The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration - PubMed (original) (raw)
The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration
Danica Chen et al. Exp Gerontol. 2008 Dec.
Abstract
A central focus of aging research is to determine how calorie restriction (CR) extends lifespan and delays diseases of aging. SIRT1, the mammalian ortholog of Sir2 in yeast, is a longevity factor which mediates dietary restriction in diverse species. In addition, SIRT1 plays a protective role in several models of neurodegenerative disease. We tested the role of SIRT1 in mediating the effects of CR in a mouse model of prion disease. Prion diseases are protein misfolding disorders of the central nervous system with many similarities to other neurodegenerative diseases, including deposition of aggregated protein, gliosis, and loss of synapses and neurons. We report that the onset of prion disease is delayed by CR and in the SIRT1 KO mice fed ad libitum. CR exerts no further effect on the SIRT1 KO strain, suggesting the effects of CR and SIRT1 deletion are mechanistically coupled. In conjunction, SIRT1 is downregulated in certain brain regions of CR mice. The expression of PrP mRNA and protein is reduced in the brains of CR mice and in SIRT1 knockout mice, suggesting a possible mechanism for the delayed onset of disease, as PrP levels are a critical determinant of how quickly mice succumb to prion disease. Surprisingly, CR greatly shortens the duration of clinical symptoms of prion disease and ultimately shortens lifespan of prion-inoculated mice in a manner that is independent of SIRT1. Taken together, our results suggest a more complex interplay between CR, SIRT1, and neurodegenerative diseases than previously appreciated.
Figures
Figure 1
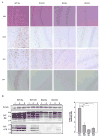
The onset of prion disease is delayed in calorie restricted mice and SIRT1 knockout mice. Wild type (WT) mice and SIRT1 KO mice fed AL or on CR diets were injected with RML prions. Brains were removed 4 months post inoculation. (A) Brain sections were stained with hematoxylin and eosin to visualize vacuolation, anti-GFAP to visualize gliosis, anti-IBA1 staining to visualize microglia, and anti-PrP (SAF) on formic acid treated samples to visualize aggregates of PrP. Scale bars correspond to 200um. (B) Brain homogenates were subjected to proteinase-K digestion. Proteinase resistant PrP was detected by western blotting with anti-PrP antibody. β-tubulin (from undigested homogenates) was used as a loading control. The level of PK-resistant PrP was quantitated using ImageJ software.
Figure 2
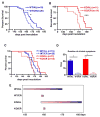
The effect of CR and SIRT1 knockout on the lifespan of mice with prion disease. (A) The survival of WT mice fed AL or CR and injected intracranially with RML prions (P=0.012, log rank test). (B) The survival of SIRT1 KO mice fed AL or CR injected intracranially with RML prion (P=0.002, log rank test). (C) The survival of WT and SIRT1 KO mice fed AL or CR injected intracranially with RML prions (combination of panels A and B). (D) The duration of clinical symptoms was shortened by CR independently of SIRT1. (E) Summary of the progression of prion disease for WT and SIRT1 KO fed AL or on CR. The beginning of each bar represents the onset of clinical symptoms and the end of each bar represents the terminal stage of the disease.
Figure 3
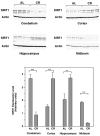
PrP expression is downregulated in CR mice and SIRT1 knockout mice. (A) PrP mRNA in brain and liver and (B) PrP protein levels in brain are downregulated in CR mice and SIRT1 KO mice. PrP mRNA and protein expression levels were compared between WT mice and SIRT1 KO mice fed AL, or WT mice fed AL or calorie restricted. mRNA levels were quantified by real time PCR and relative abundance of mRNA was obtained by normalization to cyclophilin levels (n=4). Protein levels were quantified by western blotting with anti-PrP antibody SAF83. Both tubulin and actin were used as loading controls. (C) SIRT1 promotes the expression of the PrP promoter. 293T cells were transfected with a luciferase reporter driven by the PrP promoter, with or without addition of a SIRT1 construct and/or resveratrol. The expression from the reporter construct was quantified by a luciferase assay.
Figure 4
SIRT1 is downregulated in certain brain regions of CR mice. The cerebellum, cortex, hippocampus and midbrain were dissected from WT C57Bl/6 mice fed AL or on CR (provided by the National Institute on Aging). The expression of SIRT1 in different brain regions was quantified by western blotting with anti-SIRT1 antibody using ImageJ. Actin was used as a loading control.
Similar articles
- Is Sirt1 a miracle bullet for longevity?
Imai S. Imai S. Aging Cell. 2007 Dec;6(6):735-7. doi: 10.1111/j.1474-9726.2007.00344.x. Epub 2007 Oct 17. Aging Cell. 2007. PMID: 17941969 - Tissue-specific regulation of SIRT1 by calorie restriction.
Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Chen D, et al. Genes Dev. 2008 Jul 1;22(13):1753-7. doi: 10.1101/gad.1650608. Epub 2008 Jun 11. Genes Dev. 2008. PMID: 18550784 Free PMC article. - SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice.
Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. Mercken EM, et al. Aging Cell. 2014 Feb;13(1):193-6. doi: 10.1111/acel.12151. Epub 2013 Nov 19. Aging Cell. 2014. PMID: 23941528 Free PMC article. - Sirtuins in Brain Aging and Neurological Disorders.
Qadir MI, Anwar S. Qadir MI, et al. Crit Rev Eukaryot Gene Expr. 2017;27(4):321-329. doi: 10.1615/CritRevEukaryotGeneExpr.2017019532. Crit Rev Eukaryot Gene Expr. 2017. PMID: 29283326 Review. - Calorie restriction--the SIR2 connection.
Guarente L, Picard F. Guarente L, et al. Cell. 2005 Feb 25;120(4):473-82. doi: 10.1016/j.cell.2005.01.029. Cell. 2005. PMID: 15734680 Review.
Cited by
- NMN Alleviates NP-Induced Learning and Memory Impairment Through SIRT1 Pathway in PC-12 Cell.
Li Z, Liu H, Han W, Zhu S, Liu C. Li Z, et al. Mol Neurobiol. 2023 May;60(5):2871-2883. doi: 10.1007/s12035-023-03251-9. Epub 2023 Feb 6. Mol Neurobiol. 2023. PMID: 36745337 - Antinociceptive effects of caloric restriction on post-incisional pain in nonobese rats.
Liu Y, Ni Y, Zhang W, Sun YE, Ma Z, Gu X. Liu Y, et al. Sci Rep. 2017 May 11;7(1):1805. doi: 10.1038/s41598-017-01909-8. Sci Rep. 2017. PMID: 28496116 Free PMC article. - The developing, aging neocortex: how genetics and epigenetics influence early developmental patterning and age-related change.
Huffman K. Huffman K. Front Genet. 2012 Oct 17;3:212. doi: 10.3389/fgene.2012.00212. eCollection 2012. Front Genet. 2012. PMID: 23087707 Free PMC article. - Treatment of SMB-S15 Cells with Resveratrol Efficiently Removes the PrP(Sc) Accumulation In Vitro and Prion Infectivity In Vivo.
Wang J, Zhang BY, Zhang J, Xiao K, Chen LN, Wang H, Sun J, Shi Q, Dong XP. Wang J, et al. Mol Neurobiol. 2016 Oct;53(8):5367-76. doi: 10.1007/s12035-015-9464-z. Epub 2015 Oct 6. Mol Neurobiol. 2016. PMID: 26440667 - SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus.
Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. Satoh A, et al. J Neurosci. 2010 Jul 28;30(30):10220-32. doi: 10.1523/JNEUROSCI.1385-10.2010. J Neurosci. 2010. PMID: 20668205 Free PMC article.
References
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. - PubMed
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. - PubMed
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. - PubMed
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials