Centronuclear (myotubular) myopathy - PubMed (original) (raw)
Review
Centronuclear (myotubular) myopathy
Heinz Jungbluth et al. Orphanet J Rare Dis. 2008.
Abstract
Centronuclear myopathy (CNM) is an inherited neuromuscular disorder characterised by clinical features of a congenital myopathy and centrally placed nuclei on muscle biopsy.The incidence of X-linked myotubular myopathy is estimated at 2/100000 male births but epidemiological data for other forms are not currently available.The clinical picture is highly variable. The X-linked form usually gives rise to a severe phenotype in males presenting at birth with marked weakness and hypotonia, external ophthalmoplegia and respiratory failure. Signs of antenatal onset comprise reduced foetal movements, polyhydramnios and thinning of the ribs on chest radiographs; birth asphyxia may be the present. Affected infants are often macrosomic, with length above the 90th centile and large head circumference. Testes are frequently undescended. Both autosomal-recessive (AR) and autosomal-dominant (AD) forms differ from the X-linked form regarding age at onset, severity, clinical characteristics and prognosis. In general, AD forms have a later onset and milder course than the X-linked form, and the AR form is intermediate in both respects.Mutations in the myotubularin (MTM1) gene on chromosome Xq28 have been identified in the majority of patients with the X-linked recessive form, whilst AD and AR forms have been associated with mutations in the dynamin 2 (DNM2) gene on chromosome 19p13.2 and the amphiphysin 2 (BIN1) gene on chromosome 2q14, respectively. Single cases with features of CNM have been associated with mutations in the skeletal muscle ryanodine receptor (RYR1) and the hJUMPY (MTMR14) genes.Diagnosis is based on typical histopathological findings on muscle biopsy in combination with suggestive clinical features; muscle magnetic resonance imaging may complement clinical assessment and inform genetic testing in cases with equivocal features. Genetic counselling should be offered to all patients and families in whom a diagnosis of CNM has been made.The main differential diagnoses include congenital myotonic dystrophy and other conditions with severe neonatal hypotonia.Management of CNM is mainly supportive, based on a multidisciplinary approach. Whereas the X-linked form due to MTM1 mutations is often fatal in infancy, dominant forms due to DNM2 mutations and some cases of the recessive BIN1-related form appear to be associated with an overall more favourable prognosis.
Figures
Figure 1
Male infant with X-linked centronuclear ("myotubular") myopathy due to a mutation in the myotubularin (MTM1) gene. Note generalised hypotonia and myopathic facial appearance with elongated face and inverted V-shaped mouth. (Reproduced from MedLink®Neurology, with permission)
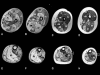
Figure 2
Muscle biopsy from the quadriceps taken at 3 months of age from a girl with X-linked centronuclear ("myotubular") myopathy due to a mutation in the myotubularin (MTM1) gene and extremely skewed X-inactivation, H&E stain, transverse section. Note marked variability in fibre size, moderate increase in connective tissue and numerous central nuclei.
Figure 3
Selective muscle involvement in a 59-year-old man (A, B, E, F) and his 28-year-old daughter with centronuclear myopathy (C, D, G, H) due to a mutation in the dynamin 2 (DNM2) gene, muscle MRI, transverse, T1-weighted sections from the proximal (A, C) and distal (B, D) thigh and the proximal (E, G) and distal lower leg (F, H). In the thigh there is increased signal intensity within the adductor longus (AL), semimembranosus (SM), rectus femoris (RF), biceps femoris (BF), and vastus intermedius (VI) muscles with relative sparing of the adductor magnus (AM), gracilis (G), sartorius (S), semitendinosus (ST), vastus lateralis (VL), and vastus medialis (VM) muscles. Within the lower leg, there is predominant involvement of the gastrocnemius (GA), soleus (SO) an tibialis anterior (TA) muscles with relative sparing of the peroneal group (PG). Muscle involvement, particularly within the thigh, is milder in the daughter compared to her father. The pattern is distinct from that reported in congenital myopathies associated with mutations in the skeletal muscle ryanodine (RYR1) gene. (Figure courtesy of Dr Carsten Boennemann and Dr Joachim Schessl, reproduced from Schessl et al., Neuromuscular Disorders 2007; 17:28–32, with permission from Elsevier).
Similar articles
- Adult centronuclear myopathies: A hospital-based study.
Echaniz-Laguna A, Biancalana V, Böhm J, Tranchant C, Mandel JL, Laporte J. Echaniz-Laguna A, et al. Rev Neurol (Paris). 2013 Aug-Sep;169(8-9):625-31. doi: 10.1016/j.neurol.2012.12.006. Epub 2013 Aug 9. Rev Neurol (Paris). 2013. PMID: 23938035 - Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies.
Toussaint A, Cowling BS, Hnia K, Mohr M, Oldfors A, Schwab Y, Yis U, Maisonobe T, Stojkovic T, Wallgren-Pettersson C, Laugel V, Echaniz-Laguna A, Mandel JL, Nishino I, Laporte J. Toussaint A, et al. Acta Neuropathol. 2011 Feb;121(2):253-66. doi: 10.1007/s00401-010-0754-2. Epub 2010 Oct 7. Acta Neuropathol. 2011. PMID: 20927630 - "Necklace" fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy.
Bevilacqua JA, Bitoun M, Biancalana V, Oldfors A, Stoltenburg G, Claeys KG, Lacène E, Brochier G, Manéré L, Laforêt P, Eymard B, Guicheney P, Fardeau M, Romero NB. Bevilacqua JA, et al. Acta Neuropathol. 2009 Mar;117(3):283-91. doi: 10.1007/s00401-008-0472-1. Epub 2008 Dec 16. Acta Neuropathol. 2009. PMID: 19084976 - Centronuclear myopathies: a widening concept.
Romero NB. Romero NB. Neuromuscul Disord. 2010 Apr;20(4):223-8. doi: 10.1016/j.nmd.2010.01.014. Epub 2010 Feb 23. Neuromuscul Disord. 2010. PMID: 20181480 Review. - X-linked myotubular and centronuclear myopathies.
Pierson CR, Tomczak K, Agrawal P, Moghadaszadeh B, Beggs AH. Pierson CR, et al. J Neuropathol Exp Neurol. 2005 Jul;64(7):555-64. doi: 10.1097/01.jnen.0000171653.17213.2e. J Neuropathol Exp Neurol. 2005. PMID: 16042307 Review.
Cited by
- Spectrum of Clinical Features in X-Linked Myotubular Myopathy Carriers: An International Questionnaire Study.
Reumers SFI, Braun F, Spillane JE, Böhm J, Pennings M, Schouten M, van der Kooi AJ, Foley AR, Bönnemann CG, Kamsteeg EJ, Erasmus CE, Schara-Schmidt U, Jungbluth H, Voermans NC. Reumers SFI, et al. Neurology. 2021 Aug 3;97(5):e501-e512. doi: 10.1212/WNL.0000000000012236. Epub 2021 May 19. Neurology. 2021. PMID: 34011573 Free PMC article. - Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy.
Dowling JJ, Low SE, Busta AS, Feldman EL. Dowling JJ, et al. Hum Mol Genet. 2010 Jul 1;19(13):2668-81. doi: 10.1093/hmg/ddq153. Epub 2010 Apr 16. Hum Mol Genet. 2010. PMID: 20400459 Free PMC article. - Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis.
Lucas L, Cooper TA. Lucas L, et al. Int J Mol Sci. 2023 Feb 2;24(3):2903. doi: 10.3390/ijms24032903. Int J Mol Sci. 2023. PMID: 36769228 Free PMC article. Review. - The Genetic Background of Abnormalities in Metabolic Pathways of Phosphoinositides and Their Linkage with the Myotubular Myopathies, Neurodegenerative Disorders, and Carcinogenesis.
Derkaczew M, Martyniuk P, Hofman R, Rutkowski K, Osowski A, Wojtkiewicz J. Derkaczew M, et al. Biomolecules. 2023 Oct 19;13(10):1550. doi: 10.3390/biom13101550. Biomolecules. 2023. PMID: 37892232 Free PMC article. Review. - Three novel MTM1 pathogenic variants identified in Japanese patients with X-linked myotubular myopathy.
Nishikawa A, Iida A, Hayashi S, Okubo M, Oya Y, Yamanaka G, Takahashi I, Nonaka I, Noguchi S, Nishino I. Nishikawa A, et al. Mol Genet Genomic Med. 2019 May;7(5):e621. doi: 10.1002/mgg3.621. Epub 2019 Mar 18. Mol Genet Genomic Med. 2019. PMID: 30884204 Free PMC article.
References
- McKusick VA. Mendelian Inheritance in Man A Catalog of Human Genes and Genetic Disorders. 12. Baltimore, London: The Johns Hopkins University Press; 1998.
- Spiro AJ, Shy GM, Gonatas NK. Myotubular myopathy. Archives of Neurology. 1966;14:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials