Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse - PubMed (original) (raw)
Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse
M C Arrieta et al. Gut. 2009 Jan.
Abstract
Background: Defects in the small intestinal epithelial barrier have been associated with inflammatory bowel disease but their role in the causation of disease is still a matter of debate. In some models of disease increased permeability appears to be a very early event. The interleukin 10 (IL10) gene-deficient mouse spontaneously develops colitis after 12 weeks of age. These mice have been shown to have increased small intestinal permeability that appears early in life. Furthermore, the development of colitis is dependent upon luminal agents, as animals do not develop disease if raised under germ-free conditions.
Aims: To determine if the elevated small bowel permeability can be prevented, and if by doing so colonic disease is prevented or attenuated.
Methods: IL10 gene-deficient (IL10(-)/(-)) mice) were treated with AT-1001 (a zonulin peptide inhibitor), a small peptide previously demonstrated to reduce small intestinal permeability. Small intestinal permeability was measured, in vivo, weekly from 4 to 17 weeks of age. Colonic disease was assessed at 8 weeks in Ussing chambers, and at 17 weeks of age inflammatory cytokines and myeloperoxidase were measured in the colon. Colonic permeability and histology were also endpoints.
Results: Treated animals showed a marked reduction in small intestinal permeability. Average area under the lactulose/mannitol time curve: 5.36 (SE 0.08) in controls vs 3.97 (SE 0.07) in the high-dose AT-1001 group, p<0.05. At 8 weeks of age there was a significant reduction of colonic mucosal permeability and increased electrical resistance. By 17 weeks of age, secretion of tumour necrosis factor alpha (TNFalpha) from a colonic explant was significantly lower in the treated group (25.33 (SE 4.30) pg/mg vs 106.93 (SE 17.51) pg/ml in controls, p<0.01). All other markers also demonstrated a clear reduction of colitis in the treated animals. Additional experiments were performed which demonstrated that AT-1001 was functionally active only in the small intestine.
Conclusions: This work suggests that increased intestinal permeability may be an important aetiological event in the development of colitis in IL10(-)/(-) mice.
Conflict of interest statement
Competing interests: JM is a member of the scientific advisory board for Alba Therapeutics. The other authors do not have competing interests.
Figures
Figure 1. Increased small intestinal permeability precedes colonic disease. (A) small intestinal permeability measurements from 4 to 17 weeks in interleukin 10 gene-deficient (IL10−/−) mice (closed circles) and Sv/Ev 129 wild-type mice (open circles) show a marked difference between these animal models very early in age. (n = 10–13 at each time point). (B) Concentration of interferon γ (IFNγ) in the small intestine of IL10−/− (closed circles) and wild-type mice (open circles) at 4, 8 and 17 weeks of age. (C) Concentration of tumour necrosis factor α (TNFα) in the small intestine of IL10−/− (closed circles) and wild-type mice (open circles) at 4, 8 and 17 weeks of age. For both cytokines, a significant difference was observed between the IL10−/− and wild-type mice only at 17 weeks. (p<0.001; n = 4–7).
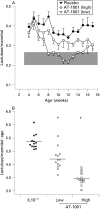
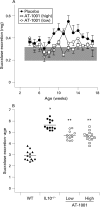
Figure 2. Small intestinal permeability is reduced with a zonulin antagonist AT-1001. (A) Small intestinal permeability was measured in IL10−/− mice treated with high dose AT-1001 (open squares), low dose (open circles) or placebo (closed circles) from 4 to 17 weeks of age. The shaded area represents the mean (3 SD) of the lactulose/mannitol ratios observed in wild-type mice. Mice treated with the high dose of AT-1001 eventually reached the wild-type range. (B) Statistical analysis of the areas under the curve of small intestinal permeabilities for each group. The permeabilities of the groups treated with AT-1001 differed significantly from the placebo group; p<0.05 (low dose), p<0.01 (high dose), n = 10–13.
Figure 7. (A) Histological scoring. The interleukin 10 gene-deficient (IL10−/−) mice and the animals treated with a low dose of the zonulin peptide inhibitor AT-1001 had significantly increased histological scores as compared to the wild-type mice (*p<0.05). However, the group treated with a high dose of AT-1001 had a histological score significantly lower than the untreated IL10−/− animals (**p<0.05), but this was still elevated significantly from the wild-type (WT) controls (n = 4–6). (B) Representative histology. All panels show colonic histology at 17 weeks of age. The upper panels (a, c, e, g) were taken at ×20 magnification, while the lower panels (b, d, f, h) are the same regions at ×40. Panels a/b represent tissue from the wild-type animals while panels c/d are from the IL10−/− mice. A clear inflammatory infiltrate is observed in the lamina propria of these animals. Panels e/f are from the low-dose group while g/h are from the high-dose treated animals. It can be appreciated that there is a significant reduction in the inflammatory component of the lesion observed in these animals.
Figure 3. The zonulin peptide inhibitor AT-1001 reduced small intestinal permeability by 8 weeks. (A) Mannitol flux was measured in Ussing chambers at 8 weeks of age and was increased in the interleukin 10 gene-deficient (IL10−/−) mice (p<0.05). This increase was not observed in animals treated with AT-1001 (n = 4–8). (B) The electrical resistance was reduced in the IL10−/− mice only (p<0.01). AT-1001 treatment prevented this reduction (n = 5–8). WT, wild-type mice.
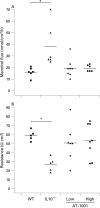
Figure 4. Attenuation of colonic permeability at 8 weeks. (A) Colonic mannitol flux was increased in the interleukin 10 gene-deficient (IL10−/−) mice (p<0.05). This increase was prevented by the zonulin peptide inhibitor AT-1001 (n = 5–8). (B) Electrical resistance was reduced in the IL10−/− mice and again prevented by AT-1001 (p<0.05, n = 5–8). WT, wild-type mice.
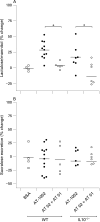
Figure 5. The zonulin peptide inhibitor AT-1001 attenuates colonic damage. (A) Sucralose excretion was measured weekly in interleukin 10 gene-deficient (IL10−/−) mice treated with a high dose of AT-1001 (open squares), low dose (open circles) or placebo (closed circles) from 4 to 17 weeks of age. The data are shown relative to that from wild-type (WT) animals, which is represented in the shaded area. As noted in the text colonic damage becomes evident at 10 weeks of age but is reduced in AT-1001 animals. (B) Statistical analysis of the areas under the curve of sucralose excretion for each group. The sucralose excretion of IL10−/− mice was significantly higher than that from wild-type animals (*p<0.01). Sucralose excretion of the animals treated with AT-1001 differed significantly from that of the IL10−/− mice (**p<0.05, n = 10–13), suggesting that AT-1001 reduces but does not prevent colonic damage.
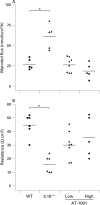
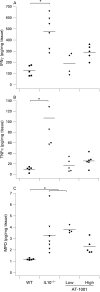
Figure 6. Colonic secretion of interferon γ (IFNγ), tumour necrosis factor α (TNFα) and myeloperoxidase (MPO) content at 17 weeks. (A and B) The interleukin 10 gene-deficient (IL10−/−) mice had a significant increase in colonic secretion of these cytokines (p<0.05). This increase was prevented by treatment with the zonulin peptide inhibitor AT-1001 (n = 4–6). (C) The IL10−/− mice and the mice treated with the low dose had a significant increase in MPO content (p<0.05). High-dose treatment of AT-1001 prevented this increase (n = 4–6).
Figure 8. Zonulin pathway mediated changes in permeability. (A) Mice were treated with the zonulin peptide agonist AT-1002 (1 mg/ml) or a mixture of AT-1002 and the zonulin peptide inhibitor AT-1001 (1 mg/ml each) for 24 h. The zonulin agonist increased small intestinal permeability (lactulose/mannitol ratio). The concomitant administration of the agonist (AT 01 + AT 02) abrogated this increase. The same effect was demonstrated in IL10−/− animals. Bovine serum albumin (BSA) was used as a protein control (p<0.05; n = 4–12). (B) Colonic permeability (sucralose excretion) was determined in the same animals. In contrast to small intestinal permeability, stimulation of the zonulin pathway had no effect in the colon of either wild-type or IL10−/− mice (4–12).
Comment in
- Gut permeability and colitis.
Mannon P. Mannon P. Gastroenterology. 2009 Aug;137(2):732-4. doi: 10.1053/j.gastro.2009.06.026. Epub 2009 Jun 28. Gastroenterology. 2009. PMID: 19567237 No abstract available.
Similar articles
- Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model.
Sturgeon C, Lan J, Fasano A. Sturgeon C, et al. Ann N Y Acad Sci. 2017 Jun;1397(1):130-142. doi: 10.1111/nyas.13343. Epub 2017 Apr 19. Ann N Y Acad Sci. 2017. PMID: 28423466 Free PMC article. - Increasing small intestinal permeability worsens colitis in the IL-10-/- mouse and prevents the induction of oral tolerance to ovalbumin.
Arrieta MC, Madsen KL, Field CJ, Meddings JB. Arrieta MC, et al. Inflamm Bowel Dis. 2015 Jan;21(1):8-18. doi: 10.1097/MIB.0000000000000253. Inflamm Bowel Dis. 2015. PMID: 25517593 - Composition of the Intestinal Microbiota Determines the Outcome of Virus-Triggered Colitis in Mice.
Bolsega S, Basic M, Smoczek A, Buettner M, Eberl C, Ahrens D, Odum KA, Stecher B, Bleich A. Bolsega S, et al. Front Immunol. 2019 Jul 23;10:1708. doi: 10.3389/fimmu.2019.01708. eCollection 2019. Front Immunol. 2019. PMID: 31396223 Free PMC article. - Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments.
Valitutti F, Fasano A. Valitutti F, et al. Dig Dis Sci. 2019 Jul;64(7):1748-1758. doi: 10.1007/s10620-019-05646-y. Dig Dis Sci. 2019. PMID: 31076989 Free PMC article. Review. - The Therapeutic use of the Zonulin Inhibitor AT-1001 (Larazotide) for a Variety of Acute and Chronic Inflammatory Diseases.
Troisi J, Venutolo G, Terracciano C, Carri MD, Di Micco S, Landolfi A, Fasano A. Troisi J, et al. Curr Med Chem. 2021;28(28):5788-5807. doi: 10.2174/0929867328666210104110053. Curr Med Chem. 2021. PMID: 33397225 Review.
Cited by
- Dietary Methionine Supplementation Exacerbates Gastrointestinal Toxicity in a Mouse Model of Abdominal Irradiation.
Ewing LE, Skinner CM, Pathak R, Yee EU, Krager K, Gurley PC, Melnyk S, Boerma M, Hauer-Jensen M, Koturbash I. Ewing LE, et al. Int J Radiat Oncol Biol Phys. 2021 Feb 1;109(2):581-593. doi: 10.1016/j.ijrobp.2020.09.042. Epub 2020 Sep 28. Int J Radiat Oncol Biol Phys. 2021. PMID: 33002540 Free PMC article. - Molecular Changes in the Non-Inflamed Terminal Ileum of Patients with Ulcerative Colitis.
Lee HS, Vancamelbeke M, Verstockt S, Wilms T, Verstockt B, Sabino J, Ferrante M, Vermeire S, Cleynen I. Lee HS, et al. Cells. 2020 Jul 28;9(8):1793. doi: 10.3390/cells9081793. Cells. 2020. PMID: 32731480 Free PMC article. - Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells.
Maria-Ferreira D, Nascimento AM, Cipriani TR, Santana-Filho AP, Watanabe PDS, Sant Ana DMG, Luciano FB, Bocate KCP, van den Wijngaard RM, Werner MFP, Baggio CH. Maria-Ferreira D, et al. Sci Rep. 2018 Aug 16;8(1):12261. doi: 10.1038/s41598-018-30526-2. Sci Rep. 2018. PMID: 30115942 Free PMC article. - Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation.
Martínez-Sánchez LDC, Ngo PA, Pradhan R, Becker LS, Boehringer D, Soteriou D, Kubankova M, Schweitzer C, Koch T, Thonn V, Erkert L, Stolzer I, Günther C, Becker C, Weigmann B, Klewer M, Daniel C, Amann K, Tenzer S, Atreya R, Bergo M, Brakebusch C, Watson AJM, Guck J, Fabry B, Atreya I, Neurath MF, López-Posadas R. Martínez-Sánchez LDC, et al. Gut. 2023 Feb;72(2):275-294. doi: 10.1136/gutjnl-2021-325520. Epub 2022 Mar 3. Gut. 2023. PMID: 35241625 Free PMC article. - The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease.
Vanuytsel T, Vermeire S, Cleynen I. Vanuytsel T, et al. Tissue Barriers. 2013 Dec 1;1(5):e27321. doi: 10.4161/tisb.27321. Epub 2013 Dec 10. Tissue Barriers. 2013. PMID: 24868498 Free PMC article. Review.
References
- Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. Ann Int Med 1986;105:883–5 - PubMed
- Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn’s disease and their healthy relatives [see comments]. Gastroenterology 1989;97:927–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials