Reversibility of aberrant global DNA and estrogen receptor-alpha gene methylation distinguishes colorectal precancer from cancer - PubMed (original) (raw)
Reversibility of aberrant global DNA and estrogen receptor-alpha gene methylation distinguishes colorectal precancer from cancer
Rulong Shen et al. Int J Clin Exp Pathol. 2009.
Abstract
Alterations in the global methylation of DNA and in specific regulatory genes are two epigenetic alterations found in cancer. However, the significance of epigenetic changes for diagnosis and/or prognosis of colorectal cancer have not been established, although it has been extensively investigated. Recently we have identified a new type of cancer cell called precancerous stem cells (pCSCs) and proposed that cancer may arise from a lengthy development process of tumor initiating cells (TICs) --> pCSCs --> cancer stem cells (CSCs) --> cancer, which is in parallel to histological changes of hyperplasia (TICs) --> precancer (pCSCs) --> carcinoma (CSCs/cancer cells), accompanied by clonal evolutionary epigenetic and genetic alterations. In this study, we investigated whether aberrant DNA methylation can be used as a biomarker for the differentiation between premalignant and malignant lesions in the colorectum. The profile of global DNA and estrogen receptor (ER)-alpha gene methylation during cancer development was determined by analysis of 5-methylcytosine (5-MeC) using immunohistochemical (IHC) staining, dot blot analysis or a quantitative gene methylation assay (QGMA). Herein we show that global DNA hypomethylation and ER-alpha gene hypermethylation are progressively enhanced from hyperplastic polyps (HPs) --> adenomatous polyps (APs) --> adenomatous carcinoma (AdCa). The aberrant methylation can be completely reversed in APs, but not in AdCa by a nonsteroidal anti-inflammatory drug (NSAID) celecoxib, which is a selective inhibitor of cyclooxygenase-2 (Cox-2), suggesting that the epigenetic alterations between colorectal precancer (AP) and cancer (AdCa) are fundamentally different in response to anti-cancer therapy. In normal colorectal mucosa, while global DNA methylation was not affected by aging, ER-alpha gene methylation was significantly increased with aging. However, this increase did not reach the level observed in colorectal APs. Taken together, reversibility of aberrant global DNA and ER-alpha gene methylation distinguishes colorectal precancer from cancer.
Keywords: DNA methylation; Precancer; cancer progression; colorectal cancer; epigenetic; estrogen receptor-α; nonsteroidal anti-inflammatory drugs; tumor initiation.
Figures
Figure 1
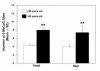
Hypomethylation of global DNA in adenomatous carcinomas. The 5-MeC in DNA of various stages of colorectal lesions was detected by IHC staining. Sections of HPs, APs, AdCas and histologically “normal” mucosa around colorectal cancers were stained with mAb to 5-MeC and counterstained with hematoxylin. The intensity of the staining was scored as described in Materials and Methods. A. Micrographs of representative staining for 5-MeC with different scores at low (×72) and high (×400) magnification: N (> 3 ∼ 4+), the section of “normal” mucosa distal to or near cancer were stained uniformly and intensely except for a few cells that were not stained uniformly or intensively (arrow); HP (3+), the hyperplastic polyps were stained as intensively but less uniformly (arrows) as “normal mucosa”; AP (2+), the adenomatous polyps were stained weakly or moderately with speckles; and AdCa (< 1+), the adenocarcinomas were essentially not stained. Note that the infiltrated mononuclear cells in the AdCa were positive for 5-MeC. The boxes in the micrographs of ×72 magnification are shown as the micrographs of ×400 magnification. B. Summary of all specimens: Normal, near: n = 49; HPs: n = 15; APs, n = 13, and AdCas: n = 21. **, p < 0.01, as compared to HPs.
Figure 2
Dot-blot analysis of global DNA methylation in various types of colorectal lesions. DNA was isolated from “normal” mucosa distal to or near cancer, HPs, APs, and AdCas, and blotted onto membranes that were probed with the monoclonal primary antibody specific for 5-MeC. The intensity of the blots was normalized by the intensity of the methylene blue staining of DNA, and the results are expressed as arbitrary units (means ± SE). Normal, distal: n = 49; Normal, near: n = 49; HPs: n = 15; APs, n = 13, and AdCas: n = 21. **, p < 0.05, as APs or AdCas compared to normal, distal; * < 0.05, as compared between APs and AdCas.
Figure 3
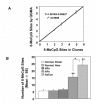
Hypermethylation of ER-α gene in adenomatous carcinomas. A. The accuracy of the Quantitative Gene Methylation Assay (QGMA) for ER-α gene. Clones containing 0, 2, 3 and 5 methylated CpG were subjected to the QGMA assay and sequencing. The number of CpG sites determined by the QGMA assay is plotted versus the number determined by sequencing. Results are means ± SE for six determinations. For the regression line, r2 = 9908. B. ER-α gene methylation during colorectal neoplastic progression. DNA was isolated from paraffin-embedded tissue sections of normal mucosa, either distal or adjacent to the lesions, HPs, APs and AdCas. After bisulfite-treatment, the DNA was amplified by PCR for the ER-α gene and assayed by QGMA. Results are expressed as the mean number of methylated CpG sites ± SE. The number of specimens used for each group is the same as in Figure 2. **, p < 0.01, as compared to normal, distal, normal, near, or HPs; *, p < 0.01, as compared between APs and AdCas.
Figure 4
The effect of aging on ER-α gene methylation in normal-appearing colorectal mucosa. DNA samples of normal colorectal mucosa distal to and near the colorectal carcinomas were further divided into two age groups, i.e., younger (n = 24) and older (n = 25) than 50 years of age. After bisulfite-treatment, the DNA was amplified by PCR for the ER-α gene and assayed by QGMA. Results are expressed as means ± SE. The asterisks indicate significant difference between the two age groups with p-value <0.01.
Figure 5
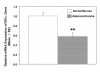
Hypermethylation of ER-α gene inhibits transcription of ER-α gene in colorectal adenocarcinomas. Total RNA was isolated from 8 normal mucosa and 6 adenocarcinomas, and subjected to real-time PCR analysis for ER-α gene expression as described in Materials and Methods. Results are expressed as means of relative ER-α mRNA expression units ± SE. The asterisk indicates significant difference between normal mucosa and adenocarcinomas, p-value < 0.01.
Figure 6
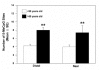
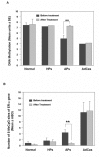
Effect of celecoxib on the methylation of global DNA and ER-α gene in colorectal tumors. Colorectal biopsies were obtained from four colorectal lesions (one hyperplastic polyp, two adenomatous polyps and one adenocarcinoma) before and after treatment of patients with 200 mg celecoxib per day for 30 days. The DNA was extracted and subjected to Dot-blot analysis and QGMA. A. Global DNA methylation was analyzed by Dot plot analysis with mAb to 5-MeC. The 5-Mec signal of DNA methylation was normalized by the equal intensity of 0.02% methylene blue staining. Results are expressed as means arbitrary units ± SE. The asterisk indicates a significant difference before and after treatment with celecoxib, p-value <0.01. B. ER-α gene methylation was analyzed by QGMA, as described in Figure 3. 10 colonies of each sample were examined for methylated CpG sites of ER-α gene, and the results are expressed as means ± SE of 5-Mec-CPG sites per ER-α gene. **, p < 0.01, as compared before and after treatment with celecoxib.
Similar articles
- Epigenetic changes (aberrant DNA methylation) in colorectal neoplasia.
Kim YS, Deng G. Kim YS, et al. Gut Liver. 2007 Jun;1(1):1-11. doi: 10.5009/gnl.2007.1.1.1. Epub 2007 Jun 30. Gut Liver. 2007. PMID: 20485652 Free PMC article. - Altered global methylation of DNA: an epigenetic difference in susceptibility for lung cancer is associated with its progression.
Piyathilake CJ, Frost AR, Bell WC, Oelschlager D, Weiss H, Johanning GL, Niveleau A, Heimburger DC, Grizzle WE. Piyathilake CJ, et al. Hum Pathol. 2001 Aug;32(8):856-62. doi: 10.1053/hupa.2001.26471. Hum Pathol. 2001. PMID: 11521231 - The relationship between gene-specific DNA methylation in leukocytes and normal colorectal mucosa in subjects with and without colorectal tumors.
Ally MS, Al-Ghnaniem R, Pufulete M. Ally MS, et al. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18(3):922-8. doi: 10.1158/1055-9965.EPI-08-0703. Epub 2009 Mar 3. Cancer Epidemiol Biomarkers Prev. 2009. PMID: 19258481 - Gene methylation in gastric cancer.
Qu Y, Dang S, Hou P. Qu Y, et al. Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review. - Beyond the island: epigenetic biomarkers of colorectal and prostate cancer.
Savio AJ, Bapat B. Savio AJ, et al. Methods Mol Biol. 2015;1238:103-24. doi: 10.1007/978-1-4939-1804-1_6. Methods Mol Biol. 2015. PMID: 25421657 Review.
Cited by
- Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker.
Liu JJ, Shen R, Chen L, Ye Y, He G, Hua K, Jarjoura D, Nakano T, Ramesh GK, Shapiro CL, Barsky SH, Gao JX. Liu JJ, et al. Int J Clin Exp Pathol. 2010 Mar 20;3(4):328-37. Int J Clin Exp Pathol. 2010. PMID: 20490325 Free PMC article. - Can precancerous stem cells be risk markers for malignant transformation in the oral mucosa?
Wang S, Ying L, Yu SY, Bai J, Hao C. Wang S, et al. Cell Mol Biol Lett. 2023 Apr 7;28(1):30. doi: 10.1186/s11658-023-00441-0. Cell Mol Biol Lett. 2023. PMID: 37029348 Free PMC article. - Expression and significance of Musashi-1 in gastric cancer and precancerous lesions.
Kuang RG, Kuang Y, Luo QF, Zhou CJ, Ji R, Wang JW. Kuang RG, et al. World J Gastroenterol. 2013 Oct 21;19(39):6637-44. doi: 10.3748/wjg.v19.i39.6637. World J Gastroenterol. 2013. PMID: 24151393 Free PMC article. - Association of estrogen receptor α gene PvuII and XbaI polymorphisms with non-small cell lung cancer.
Chang HL, Cheng YJ, Su CK, Chen MC, Chang FH, Lin FG, Liu LF, Yuan SS, Chou MC, Huang CF, Yang CC. Chang HL, et al. Oncol Lett. 2012 Feb;3(2):462-468. doi: 10.3892/ol.2011.482. Epub 2011 Nov 15. Oncol Lett. 2012. PMID: 22740932 Free PMC article. - Case-control study of candidate gene methylation and adenomatous polyp formation.
Alexander M, Burch JB, Steck SE, Chen CF, Hurley TG, Cavicchia P, Shivappa N, Guess J, Zhang H, Youngstedt SD, Creek KE, Lloyd S, Jones K, Hébert JR. Alexander M, et al. Int J Colorectal Dis. 2017 Feb;32(2):183-192. doi: 10.1007/s00384-016-2688-1. Epub 2016 Oct 22. Int J Colorectal Dis. 2017. PMID: 27771773 Free PMC article.
References
- American Cancer Society. Colorectal Cancer Fact. http://www.cancer.org/2006.
- Raju R, Cruz-Correa M. Chemoprevention of colorectal cancer. Dis Colon Rectum. 2006;49:113–124. discussion 24–25. - PubMed
- Cancer. World Health Organization. http://www.who.int/mediacentre/factsheets/f s297/en/2007.
- Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, Wen J, Zimmerer J, Wang Y, Liu Y, Lasky LC, Heerema NA, Perrotti D, Ozato K, Kuramochi-Miyagawa S, Nakano T, Yates AJ, Carson Iii WE, Lin H, Barsky SH, Gao JX. Precancerous Stem Cells Have the Potential for both Benign and Malignant Differentiation. PLoS ONE. 2007;2:e293. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous