Limbic epileptogenesis in a mouse model of fragile X syndrome - PubMed (original) (raw)
Limbic epileptogenesis in a mouse model of fragile X syndrome
Li-Feng Qiu et al. Cereb Cortex. 2009 Jul.
Abstract
Fragile X syndrome (FXS), caused by silencing of the Fmr1 gene, is the most common form of inherited mental retardation. Epilepsy is reported to occur in 20-25% of individuals with FXS. However, no overall increased excitability has been reported in Fmr1 knockout (KO) mice, except for increased sensitivity to auditory stimulation. Here, we report that kindling increased the expressions of Fmr1 mRNA and protein in the forebrain of wild-type (WT) mice. Kindling development was dramatically accelerated in Fmr1 KO mice, and Fmr1 KO mice also displayed prolonged electrographic seizures during kindling and more severe mossy fiber sprouting after kindling. The accelerated rate of kindling was partially repressed by inhibiting N-methyl-D-aspartic acid receptor (NMDAR) with MK-801 or mGluR5 receptor with 2-methyl-6-(phenylethynyl)-pyridine (MPEP). The rate of kindling development in WT was not effected by MPEP, however, suggesting that FMRP normally suppresses epileptogenic signaling downstream of metabolic glutamate receptors. Our findings reveal that FMRP plays a critical role in suppressing limbic epileptogenesis and predict that the enhanced susceptibility of patients with FXS to epilepsy is a direct consequence of the loss of an important homeostatic factor that mitigates vulnerability to excessive neuronal excitation.
Figures
Figure 1.
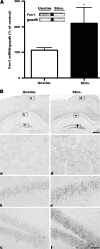
Kindling upregulates Fmr1 mRNA in the forebrain. (A) Quantitative real-time PCR (lower) and RT-PCR (upper) analysis of Fmr1 mRNA expression. Total RNA was isolated from forebrains of fully kindled WT mice 3 h after an evoked class 5 seizure (Stim.) or without any further stimulation (Unstim.). Fmr1 mRNA was normalized to G_apdh_ mRNA levels and mean ± standard error of the mean values are presented as a percentage of unstimulated controls; *P < 0.05, unpaired _t_-test. (B) Fmr1 in situ hybridization of coronal sections from unstimulated fully kindled WT mice (left panel) and fully kindled WT mice 3 h after seizure (right panel). In the unstimulated group, Fmr1 expression is relatively low in the cortex (a), the CA1 and the DG (b and c). Three hours after a class 5 seizure, Fmr1 mRNA is upregulated in both the cortex (d) and hippocampus (e and f). Lower panels show higher magnification images of the areas indicated by the boxes in the upper panels. Scale bars: upper panel, 400 μm in upper panel; lower panel, 50 μm.
Figure 2.
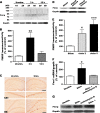
FMRP expression is upregulated after seizure activity. (A) Representative western blot showing a transient increase of FMRP in the forebrain of kindled mice after an evoked seizure. Two weeks after fully kindling, a single class 5 seizure was induced in WT mice and forebrains were isolated 3 h or 12 h later. Each lane was loaded with an equal amount of protein extract from a single forebrain sample (mouse #1–6). Lane 1 and 2, unstimulated fully kindled WT mice; lane 3 and 4, fully kindled mice 3 h after a class 5 seizure; lane 5 and 6, fully kindled mice 12 h after a class 5 seizure. After a class 5 seizure, FMRP expression increases before returning to baseline levels by 12 h. (B) Quantitative analysis of western blot band intensities. FMRP immunoreactivity was normalized to Gapdh immunoreactivity and mean ± standard error of the mean (SEM) values are presented as a percentage of the mean level in unstimulated fully kindled mice; *P < 0.05; **P < 0.01; ***P < 0.001; 1-way ANOVA followed by post hoc Dunnett's test. (C) Seizure activity leads to increased FMRP expression in the soma of CA1 pyramidal cells and granule cells in the DG. Coronal sections through the hippocampus of unstimulated fully kindled WT mice (left panel) and fully kindled WT mice 3 h after a class 5 seizure-inducing stimulation (right panel) were immunolabeled with anti-FMRP. Lower panels show higher magnification of upper panel, note that no staining is observed in neurites and nucleus of hippocampal neurons. Scale bars: 400 μm for upper 1 row; 100 μm for lower 2 rows. (D) Upregulation of FMRP correlates with kindling development. Forebrains of WT mice isolated 3 h after sham stimulation (lane 1), 3 h after the first class 2 seizure during kindling (lane 2), or 3 h after the third class 5 seizure during kindling (lane 3). (E) Quantitative analysis of FMRP expression in (D). FMRP levels were normalized to Gapdh and mean ± SEM values are presented as a percentage of the mean level in sham-stimulated mice; *P < 0.05; **P < 0.01; ***P < 0.001; 1-way ANOVA with post hoc Dunnett's test. (F) Real-time PCR of Fmr1 RNA from control mice, saline-treated stimulated mice, actinomycin D-treated stimulated mice. Fmr1 transcript levels were upregulated at 3 h after stimulation in saline-treated mice and application of actinomycin D 30 min before stimulation repressed the increase of Fmr1. Gapdh transcript levels were used to normalize the levels of Fmr1 and values are presented as group mean ± SEM as a percentage of unstimulated samples; *P < 0.05; 1-way ANOVA, post hoc Dunnett's test. (G) Representative western blot showing that application of actinomycin D did not obviously repress seizure-induced increases of FMRP.
Figure 3.
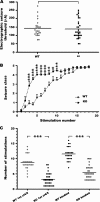
Striking acceleration of kindling development in Fmr1 KO mice. (A) No significant difference between WT and Fmr1 KO in mean EST is observed; P = 0.77, unpaired _t_-test. (B) Behavioral seizure intensities (class 1–5) evoked by amygdala stimulation at the predetermined EST, presented as mean ± SEM of WT mice (n = 25) and Fmr1 KO mice (n = 31). (C) Number of stimulations required to provoke the first episode of class 4/5 seizure and third consecutive episode of class 4/5 seizure in WT mice (n = 25) and Fmr1 KO mice (n = 31) mice; ***P < 0.001; 2-tailed unpaired _t_-test.
Figure 4.
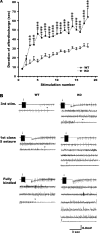
Fmr1 KO mice exhibit prolonged electrographic seizures. (A) AD durations evoked by amygdala stimulation at the predetermined EST. The progressive prolongation of AD durations was significantly accelerated in Fmr1 KO mice compared with WT mice. Data presented as mean ± standard error of the mean of WT mice (n = 25) and Fmr1 KO mice (n = 31). (B) Representative EEG recordings of WT and Fmr1 KO mice at the third stimulation, first class 5 seizure-inducing stimulation and third consecutive class 5 seizure-inducing stimulation. Arrows indicate the application of stimulation, and the arrowheads indicate the termination point of the electrographic seizure.
Figure 5.
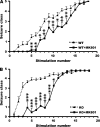
Effects of the NMDA antagonist MK801 on kindling development in WT and Fmr1 KO mice. (A) Behavioral seizure intensities evoked by amygdala stimulation at the predetermined EST, data presented as mean ± standard error of the mean (SEM) of saline-treated WT (n = 25) and MK801-treated WT mice (n = 13). (B) Behavioral seizure intensities evoked by amygdala stimulation at the predetermined EST, data presented as mean ± SEM of saline-treated (n = 31) and MK801-treated Fmr1 KO mice (n = 13). MK801 significantly slowed kindling development in both WT mice and Fmr1 KO mice. Saline or MK801 was administered 30 min before each stimulation. Asterisks indicate statistically significant differences in the average behavior seizure class at the indicated time point between the 2 presented groups. *P < 0.05; **P < 0.01; ***P < 0.001; 2-tailed unpaired _t_-test.
Figure 6.
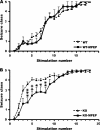
Effects of the mGluR5 antagonist MPEP on kindling development in WT and Fmr1 KO mice. (A) Behavioral seizure class evoked by amygdala stimulation at the predetermined EST, data presented as mean ± standard error of the mean (SEM) of saline-treated (n = 25) and MPEP-treated WT mice (n = 15). There is no significant difference between kindling development in WT mice treated with MPEP compared with controls. (B) Behavioral seizure class evoked by amygdala stimulation at the predetermined EST, data presented as mean ± SEM of saline-treated (n = 31) and MPEP-treated Fmr1 KO mice treated (n = 13). Administering MPEP to Fmr1 KO mice significantly represses seizure intensities at the fourth through eighth stimulation point. Saline or MK801 was administered 30 min before each stimulation. Asterisks indicate statistically significant differences in the average behavior seizure class at the indicated time point between the 2 presented groups. *P < 0.05; **P < 0.01; ***P < 0.001; 2-tailed unpaired _t_-test.
Figure 7.
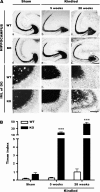
Axon projections of granule cells in the hippocampus of sham-stimulated and fully kindled WT and Fmr1 KO mice. (A) Representative Timm staining of horizontal brain sections of sham-stimulated (sham) WT mice (a, g); sham Fmr1 KO mice (d, j); fully kindled (kindled) WT mice 5 weeks (b, h) or 28 weeks (c, i) after 22 daily stimulation; kindled Fmr1 KO mice 5 weeks (e, k) or 28 weeks (f, l) after 22 daily stimulations. Under control conditions (sham), Fmr1 KO mice show slightly more Timm-stained granules in the granule cell body layer compared with WT mice. Five weeks after 22 daily stimulations, MFS is obvious in the IML of the DG in Fmr1 KO mice. Twenty-eight weeks after 22 daily stimulations, MFS is dramatically more severe in Fmr1 KO mice. In contrast, WT mice show little MFS 28 weeks after 22 daily stimulations. Scale bars: upper 2 panels, 250 μm; lower 2 panels, 50 μm. (B) Statistical analysis of Timm index in the IML of the DG. Bars represent mean ± standard error of the mean. ***P < 0.001; 2-way ANOVA with post hoc Bonferroni's test.
Figure 8.
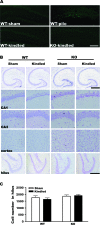
Fluoro-Jade B and Nissl staining reveal no obvious neuronal cell loss in Fmr1 KO mice after kindling. (A) Fluoro-Jade B staining. A WT hippocampal section 24 h after pilocarpine-induced seizure (340 mg/kg, intraperitoneally) with significant cell death in the hilus of the DG is shown as a positive control (n = 3). No cell death is detected in sections from sham WT (n = 3), kindled WT (n = 6), or kindled Fmr1 KO (n = 6) mice 24 h after the third consecutive class 5 seizure. Scale bar: 400 μm. (B) Nissl staining. Nissl-stained brain sections from sham WT mice, kindled WT mice, sham Fmr1 KO mice, and kindled Fmr1 KO mice reveal similar cell densities in the CA1, CA3, cortex, and hilus of the DG. Scale bars: top, 500 μm for upper one row; bottom, 250 μm for other rows. (C) Statistical analysis of cell number in the hilus. No significant difference was found among the 4 groups. Bars represent mean ± standard error of the mean; 1-way ANOVA, post hoc Dunnett's test.
Comment in
- Fragile X mental retardation protein in the driver's seat.
Brenman JE. Brenman JE. Cereb Cortex. 2009 Jul;19(7):1490-2. doi: 10.1093/cercor/bhp089. Epub 2009 Apr 22. Cereb Cortex. 2009. PMID: 19386637 Free PMC article. No abstract available. - The fragile X mental retardation protein: a valuable partner in the battle against epileptogenesis.
Merlin LR. Merlin LR. Epilepsy Curr. 2009 Jul-Aug;9(4):116-8. doi: 10.1111/j.1535-7511.2009.01311.x. Epilepsy Curr. 2009. PMID: 19693330 Free PMC article. No abstract available.
Similar articles
- Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome.
Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Spencer CM, et al. Genes Brain Behav. 2005 Oct;4(7):420-30. doi: 10.1111/j.1601-183X.2005.00123.x. Genes Brain Behav. 2005. PMID: 16176388 - Fmr1 knockout mice are impaired in a leverpress escape/avoidance task.
Brennan FX, Albeck DS, Paylor R. Brennan FX, et al. Genes Brain Behav. 2006 Aug;5(6):467-71. doi: 10.1111/j.1601-183X.2005.00183.x. Genes Brain Behav. 2006. PMID: 16923151 - BDNF and TrkB in neuronal differentiation of Fmr1-knockout mouse.
Louhivuori V, Vicario A, Uutela M, Rantamäki T, Louhivuori LM, Castrén E, Tongiorgi E, Akerman KE, Castrén ML. Louhivuori V, et al. Neurobiol Dis. 2011 Feb;41(2):469-80. doi: 10.1016/j.nbd.2010.10.018. Epub 2010 Nov 1. Neurobiol Dis. 2011. PMID: 21047554 - The fragile X-cerebellum connection.
Huber KM. Huber KM. Trends Neurosci. 2006 Apr;29(4):183-5. doi: 10.1016/j.tins.2006.02.001. Epub 2006 Feb 28. Trends Neurosci. 2006. PMID: 16500716 Review. - Fragile X syndrome and epilepsy.
Qiu LF, Hao YH, Li QZ, Xiong ZQ. Qiu LF, et al. Neurosci Bull. 2008 Oct;24(5):338-44. doi: 10.1007/s12264-008-1221-0. Neurosci Bull. 2008. PMID: 18839028 Free PMC article. Review.
Cited by
- Origins of epilepsy in fragile X syndrome.
Hagerman PJ, Stafstrom CE. Hagerman PJ, et al. Epilepsy Curr. 2009 Jul-Aug;9(4):108-12. doi: 10.1111/j.1535-7511.2009.01309.x. Epilepsy Curr. 2009. PMID: 19693328 Free PMC article. - Differential roles of α-, β-, and γ-actin in axon growth and collateral branch formation in motoneurons.
Moradi M, Sivadasan R, Saal L, Lüningschrör P, Dombert B, Rathod RJ, Dieterich DC, Blum R, Sendtner M. Moradi M, et al. J Cell Biol. 2017 Mar 6;216(3):793-814. doi: 10.1083/jcb.201604117. Epub 2017 Feb 28. J Cell Biol. 2017. PMID: 28246119 Free PMC article. - Emerging pharmacotherapies for neurodevelopmental disorders.
Wetmore DZ, Garner CC. Wetmore DZ, et al. J Dev Behav Pediatr. 2010 Sep;31(7):564-81. doi: 10.1097/DBP.0b013e3181ee3833. J Dev Behav Pediatr. 2010. PMID: 20814256 Free PMC article. Review. - Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice.
Pietropaolo S, Guilleminot A, Martin B, D'Amato FR, Crusio WE. Pietropaolo S, et al. PLoS One. 2011 Feb 22;6(2):e17073. doi: 10.1371/journal.pone.0017073. PLoS One. 2011. PMID: 21364941 Free PMC article. - Hyperexcitability and Homeostasis in Fragile X Syndrome.
Liu X, Kumar V, Tsai NP, Auerbach BD. Liu X, et al. Front Mol Neurosci. 2022 Jan 6;14:805929. doi: 10.3389/fnmol.2021.805929. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35069112 Free PMC article.
References
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. - PubMed
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
- Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. 2002;44:724–728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials