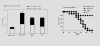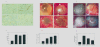Anti-angiogenesis therapy based on the bone marrow-derived stromal cells genetically engineered to express sFlt-1 in mouse tumor model - PubMed (original) (raw)
doi: 10.1186/1471-2407-8-306.
J-L Yang, H Teng, Y-Q Jia, R Wang, X-W Zhang, Y Wu, Y Luo, X-C Chen, R Zhang, L Tian, X Zhao, Y-Q Wei
Affiliations
- PMID: 18947384
- PMCID: PMC2580769
- DOI: 10.1186/1471-2407-8-306
Anti-angiogenesis therapy based on the bone marrow-derived stromal cells genetically engineered to express sFlt-1 in mouse tumor model
M Hu et al. BMC Cancer. 2008.
Abstract
Background: Bone marrow-derived stromal cells (BMSCs) are important for development, tissue cell replenishment, and wound healing in physiological and pathological conditions. BMSCs were found to preferably reach sites undergoing the process of cell proliferation, such as wound and tumor, suggesting that BMSCs may be used as a vehicle for gene therapy of tumor.
Methods: Mouse BMSCs were loaded with recombinant adenoviruses which express soluble Vascular Endothelial Growth Factor Receptor-1 (sFlt-1). The anti-angiogenesis of sFlt-1 in BMSCs was determined using endothelial cells proliferation inhibition assay and alginate encapsulation assay. The anti-tumor effects of BMSCs expressing sFlt-1 through tail-vein infusion were evaluated in two mouse tumor metastases models.
Results: BMSCs genetically modified with Adv-GFP-sFlt-1 could effectively express and secret sFlt-1. BMSCs loaded with sFlt-1 gene could preferentially home to tumor loci and decrease lung metastases and prolong lifespan in mouse tumor model through inducing anti-angiogenesis and apoptosis in tumors.
Conclusion: We demonstrated that BMSCs might be employed as a promising vehicle for tumor gene therapy which can effectively not only improve the concentration of anticancer therapeutics in tumors, but also modify the tumor microenvironment.
Figures
Figure 1
BMSCs infection and verification of secreted sFlt-1. After the BMSCs were confirmed by flow cytometric analysis, BMSCs were grown to 90% confluence and infected with adenoviruses at a multiple of infection of 3000 for 2 hours. 24 hours later, BMSCs were harvested and ready for use. Fluorescence microscope showed up to 100% GFP-positive cells. (Figure 1-A). Supernatants deposits from sFlt-1 bearing BMSCs could be recognized by antibodies reactive to NH2 terminus of mouse Flt-1, but negative staining in supernatants deposits from control BMSCs in Western blot analysis. (Figure 1-B). Conditioned media from Adv-sFlt-1 infected BMSCs was shown to inhibit the VEGF-driven mouse endothelial cells proliferation by about 50% compared with controls (Figure 1-C, P < 0.05).
Figure 2
Anti-tumor effect in mouse CT26 colon adenocarcinoma model. Female BALB/c mice received i.v. injection of 2×105 CT26 cells in 100 μl of PBS. Treatment with 1×106 Adv-GFP-sFlt-1 infected BMSCs(◆, black rhombus), 1×106 Adv-GFP infected BMSCs (▪, black quadrilateral), 1×106 uninfected BMSCs (▲, black triangle) and 100 μl of 0.9% NaCl solution alone (●, black circularity) were administered i.v. from tail vein. Treatment with sFlt-1-bearing BMSCs could decrease the number of and growth of surface metastases (Figure 2-A, D) and abolish the tumor burden by rendering the weight of lungs similar to that of normal mice (Figure 2-C). Mean numbers of lung tumor nodules in each group are shown (Figure 2-B). Values are plotted as means ± SEM (P < 0.05). The percentage of metastatic foci > 3 mm are marked as solid bars. A significant increase of survival rate in sFlt-1-bearing BMSCs treated mice, compared with the controls (Figure 2-E, P < 0.05. By log-rank test), was found in the tumor model.
Figure 3
Anti-tumor effect in mouse LLC lung carcinoma model. Female C57BL/6 mice received i.v. injection of 2×105 LLC cells in 100 μl of PBS. Treatment with 1×106 Adv-GFP-sFlt-1 infected BmSCs (◆, black rhombus), 1×106 Adv-GFP infected BmSCs (▪, black quadrilateral), 1×106 uninfected BmSCs (▲, black triangle) and 100 μl of 0.9% NaCl solution alone (●, black circularity) were administered i.v. from tail vein. A significant increase of survival and decrease of metastases in sFlt-1-expressing BMSCs treated mice, compared with the controls (P < 0.05. By log-rank test), was found in this model.
Figure 4
Evaluation of anti-angiogenetic effect by CD31 immunohistochemistry and alginate assay. Sections of frozen CT26 tumor tissues obtained from mice treated with sFlt-1-bearing BMSCs, GFP-expressing BMSCs, unmodified BmSCs and 0.9% NaCl solution were stained with CD31 antibody and Peroxidase-DAB (Figure 4-A). Vessel density was determined by counting the number of microvessels per high-power field (×200) in sections. (Figure4-B). Alginate beads containing different ratio of sFlt-1-bearing BMSCs and LLC (1/10, 1/100, 1/1000, LLC only) were implanted subcutaneously into C57BL/6 mice. Three weeks later, beads were surgically removed, and FITC-dextran was quantified. FITC-dextran uptake decrease and photograph of alginate implants showed the reduction of vascularization in beads containing relatively more sFlt-1-expressing BMSCs. (Figure4-C and D). Alginate beads containing MethA, BMSCs and mixture of both with ratio of 1/10 were prepared. Beads-MethA and beads-mixture were served as positive and negative controls. The third team of mice was implanted with two different beads containing MethA or BMSCs respectively in different sites of mice, which served as systemic sFlt-1 model to show the non-targeted effect of viruses. The fourth team of mice was implanted with beads containing MethA and hence injected intravenously with BMSCs in the same number of the former team, which served as local sFlt-1 model to show the targeted effect of viruses loaded in BMSCs. Compared with the controls, the effect of systemic production of sFlt-1 was weaker than local one. (Figure 4-E and F).
Figure 5
Apoptosis Assay by TUNEL staining. Paraffin sections from CT26 tumor tissues obtained from mice systemically administered with sFlt-1-expressing BMSCs, GFP expressing BMSCs, unmodified BMSCs and NaCl solution were stained using an in situ cell death detection kit (Roche) following the manufacturer's protocol. Apoptosis was determined by counting the number of the positive cells per high-power field in tumor sections. Original magnification, ×200. (Figure 5-A and B, P < 0.05).
Figure 6
Fluorescent in situ hybridization. Frozen sections of lung metastases were tested for the presence of Y chromosome which is indication of male originated BMSCs by Cy3 labeled Y chromosome probe (STAR*FISH; Cambio, Cambridge, England). Positive result could be seen in the tumor area obviously, whereas the relatively normal parts of the sections were totally negative for fluorescent signals.
Similar articles
- Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1.
Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, Zhao L, Qian C, Qian Q, Liu X. Zhang Z, et al. Mol Ther. 2005 Apr;11(4):553-62. doi: 10.1016/j.ymthe.2004.12.015. Mol Ther. 2005. PMID: 15771958 - Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice.
Davidoff AM, Ng CY, Brown P, Leary MA, Spurbeck WW, Zhou J, Horwitz E, Vanin EF, Nienhuis AW. Davidoff AM, et al. Clin Cancer Res. 2001 Sep;7(9):2870-9. Clin Cancer Res. 2001. PMID: 11555605 - Paracrine action of sFLT-1 secreted by stably-transfected Ehrlich ascites tumor cells and therapy using sFLT-1 inhibits ascites tumor growth in vivo.
Ramachandra S, D'Souza SS, Gururaj AE, Shaila MS, Salimath BP. Ramachandra S, et al. J Gene Med. 2009 May;11(5):422-34. doi: 10.1002/jgm.1309. J Gene Med. 2009. PMID: 19266483 - Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity.
Mahasreshti PJ, Kataram M, Wang MH, Stockard CR, Grizzle WE, Carey D, Siegal GP, Haisma HJ, Alvarez RD, Curiel DT. Mahasreshti PJ, et al. Clin Cancer Res. 2003 Jul;9(7):2701-10. Clin Cancer Res. 2003. PMID: 12855650 - Targeted delivery of mutant Raf kinase to neovessels causes tumor regression.
Hood JD, Cheresh DA. Hood JD, et al. Cold Spring Harb Symp Quant Biol. 2002;67:285-91. doi: 10.1101/sqb.2002.67.285. Cold Spring Harb Symp Quant Biol. 2002. PMID: 12858551 Review. No abstract available.
Cited by
- Immunotherapeutic organoids: a new approach to cancer treatment.
Compte M, Nuñez-Prado N, Sanz L, Alvarez-Vallina L. Compte M, et al. Biomatter. 2013 Jan-Mar;3(1):e23897. doi: 10.4161/biom.23897. Epub 2013 Jan 1. Biomatter. 2013. PMID: 23507921 Free PMC article. Review. - Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance.
Xuan X, Tian C, Zhao M, Sun Y, Huang C. Xuan X, et al. Cancer Cell Int. 2021 Nov 4;21(1):595. doi: 10.1186/s12935-021-02300-4. Cancer Cell Int. 2021. PMID: 34736460 Free PMC article. Review. - Adenoviral vector encoding soluble Flt-1 engineered human endometrial mesenchymal stem cells effectively regress endometriotic lesions in NOD/SCID mice.
Koippallil Gopalakrishnan AR, Pandit H, Metkari SM, Warty N, Madan T. Koippallil Gopalakrishnan AR, et al. Gene Ther. 2016 Jul;23(7):580-91. doi: 10.1038/gt.2016.30. Epub 2016 Mar 18. Gene Ther. 2016. PMID: 26990775 - TNFSF15 inhibits vasculogenesis by regulating relative levels of membrane-bound and soluble isoforms of VEGF receptor 1.
Qi JW, Qin TT, Xu LX, Zhang K, Yang GL, Li J, Xiao HY, Zhang ZS, Li LY. Qi JW, et al. Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):13863-8. doi: 10.1073/pnas.1304529110. Epub 2013 Aug 5. Proc Natl Acad Sci U S A. 2013. PMID: 23918400 Free PMC article. - Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype.
Hill BS, Pelagalli A, Passaro N, Zannetti A. Hill BS, et al. Oncotarget. 2017 Aug 14;8(42):73296-73311. doi: 10.18632/oncotarget.20265. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 29069870 Free PMC article. Review.
References
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. - DOI - PubMed
- Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–38. doi: 10.1002/jgm.452. - DOI - PubMed
- Koç OmerN, Gerson StantonL, Cooper BrendaW, Dyhouse StephanieM, Haynesworth StephenE, Caplan ArnoldI, Lazarus HillardM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical