Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models - PubMed (original) (raw)
. 2008 Nov 24;205(12):2781-9.
doi: 10.1084/jem.20081588. Epub 2008 Oct 27.
Guiqiong He, Philip T T Ly, Christopher J Fox, Matthias Staufenbiel, Fang Cai, Zhuohua Zhang, Shengcai Wei, Xiulian Sun, Chia-Hsiung Chen, Weihui Zhou, Ke Wang, Weihong Song
Affiliations
- PMID: 18955571
- PMCID: PMC2585842
- DOI: 10.1084/jem.20081588
Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models
Hong Qing et al. J Exp Med. 2008.
Abstract
Neuritic plaques in the brains are one of the pathological hallmarks of Alzheimer's disease (AD). Amyloid beta-protein (Abeta), the central component of neuritic plaques, is derived from beta-amyloid precursor protein (APP) after beta- and gamma-secretase cleavage. The molecular mechanism underlying the pathogenesis of AD is not yet well defined, and there has been no effective treatment for AD. Valproic acid (VPA) is one of the most widely used anticonvulsant and mood-stabilizing agents for treating epilepsy and bipolar disorder. We found that VPA decreased Abeta production by inhibiting GSK-3beta-mediated gamma-secretase cleavage of APP both in vitro and in vivo. VPA treatment significantly reduced neuritic plaque formation and improved memory deficits in transgenic AD model mice. We also found that early application of VPA was important for alleviating memory deficits of AD model mice. Our study suggests that VPA may be beneficial in the prevention and treatment of AD.
Figures
Figure 1.
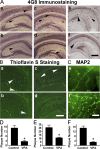
VPA treatment significantly reduces neuritic plaque formation in AD transgenic mice. (A) APP23 mice at the age of 7 or 9 mo and APP23/PS45 double-transgenic mice at the age of 6 wk were treated with 30 mg/kg VPA for 4 wk, whereas age-matched control APP23 mice received the vehicle solution. The mice were killed after behavioral tests and the brains were dissected, fixed, and sectioned. Neuritic plaques were detected using an Aβ-specific monoclonal antibody 4G8 and the DAB method. The plaques were visualized by microscopy with 40X magnification. The number of neuritic plaques was significantly reduced in VPA-treated mice compared with controls. b, d, and f show the representative brain section of the 7-mo APP23 age group, 9-mo APP23 age group, and APP23/PS45 mice treated with VPA, and a, c, and e show their controls, respectively. Black arrows point to plaques. (B) Neuritic plaques were further confirmed using thioflavin S fluorescent staining and visualized by microscopy at 40X magnification. There were less neuritic plaques in VPA-treated mice (b and d) compared with age-matched control mice (a and c). a and b show brain sections of APP23 mice, and c and d show the brain sections of APP23/PS45 mice. White arrows point to green fluorescent neuritic plaques. (C) The brain sections of APP23 were also stained with MAP-2 antibody. VPA treatment significantly increased the number of MAP-2–positive neurites. Bars: (A and B) 400 μm; (C) 200 μm. (D) Quantification of neuritic plaques in APP23 mice with treatment starting at the age of 7 mo, the number represents mean ± SEM. n = 30 mice each. *, P < 0.0001 by Student's t test. (E) Quantification of neuritic plaques in APP23 mice with treatment starting at the age of 9 mo, the number represents mean ± SEM. n = 12 mice each. *, P < 0.005 by Student's t test. (F) Quantification of neuritic plaques in APP23/PS45 mice with treatment starting at the age of 6 wk, the number represents mean ± SEM. n = 25 mice for control and 29 mice for VPA. *, P < 0.0001 by Student's t test.
Figure 2.
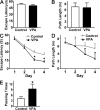
VPA improves memory deficits in AD transgenic mice. A Morris water maze test consists of 1 d of visible platform tests and 4 d of hidden platform tests, plus a probe trial 24 h after the last hidden platform test. Animal movement was tracked and recorded by HVS 2020 Plus image analyzer. The 7-mo APP23 age group mice were tested after 1 mo of daily VPA (n = 30 mice) or vehicle solution (n = 30 mice) injections. (A) During the first day of visible platform tests, the VPA-treated and control APP23 mice exhibited a similar latency to escape onto the visible platform. P > 0.05 by Student's t test. (B) The VPA-treated and control APP23 mice had similar swimming distances before escaping onto the visible platform in the visible platform test. P > 0.05 by Student's t test. (C) In hidden platform tests, mice were trained with 6 trials per day for 4 d. VPA-treated APP23 mice showed a shorter latency to escape onto the hidden platform on the third and fourth day. P < 0.001 by ANOVA. (D) The VPA-treated APP23 mice had a shorter swimming length before escaping onto the hidden platform on the third and fourth day. P < 0.01 by ANOVA. (E) In the probe trial on the sixth day, the VPA-treated APP23 mice traveled into the third quadrant, where the hidden platform was previously placed, significantly more times than controls. *, P < 0.005 by Student's t test.
Figure 3.
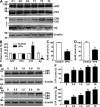
VPA inhibits γ-secretase cleavage of APP and Aβ production. (A) Half brains from VPA-treated and control APP23 mice of the 7-mo age group were lysed in RIPA-Doc lysis buffer and separated with 16% Tris-Tricine SDS-PAGE. APP full-length and CTFs (C99 and C83) were detected by C20 polyclonal antibody. PS1 was detected by anti-PS1 N-terminal antibody 231. Total Aβ was isolated from the brain tissues with 4G8 monoclonal antibody and detected using 6E10 anti-Aβ monoclonal antibody. β-Actin was detected by anti–β-actin antibody AC-15 as the internal control. (B) Quantification showed that CTFs were significantly increased, whereas Aβ levels were markedly reduced in VPA-treated mice. n = 30 each for control and VPA group. *, P < 0.05 by Student's t test. ELISA assay was performed to measure Aβ40 (C) and Aβ42 (D) levels in the conditioned media of primary neuronal cultures derived from the brain tissues of newborn APP23/PS45 mice. The cells were cultured for a week before VPA treatment for 24 h. n = 3. *, P < 0.005 by Student's t test. (E) Swedish mutant APP stable cell line 20E2 was cultured and treated with different doses of VPA for 24 h, and cell lysates were subjected to Western blot analysis. C99 and C83 were detected with C20 antibody. β-Actin was detected by anti–β-actin antibody AC-15 as the internal control. (F) Quantification of CTF (C99 and C83) generation in 20E2 cells. VPA treatment significantly increased APP CTF production. n = 4. *, P < 0.001 by ANOVA. (G) APP C99 stable cell line H99C1 was treated with different doses of VPA for 24 h, and the CTFs (C99, C89, and C83) were detected by 9E10 antibody. β-Actin was detected by anti–β-actin antibody AC-15 as the internal control. (H) Quantification of CTFs (C99, C89, and C83) levels in H99C1 cells. VPA treatment significantly increased APP CTF production. n = 4. *, P < 0.001 by ANOVA.
Figure 4.
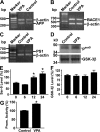
VPA inhibits GSK-3β activity. Total RNA was isolated from N2a cells by TRI-Reagent (Sigma-Aldrich). A set of gene-specific primers were used to amplify APP (A), BACE1 (B), and PS1 (C) genes. β-Actin was used as an internal control. There was no difference in endogenous APP, PS1, or BACE1 mRNA levels between VPA-treated cells and controls. (D) Brain tissues from APP23/PS45 double-transgenic mice were subjected to Western blot analysis to determine the levels of total GSK-3β and phospho–GSK-3βS9. VPA increases phospho–GSK-3βS9 (PSer9) levels, but not total GSK-3β levels in the transgenic mice. (E) N2a cells were treated with 5 mM of VPA for 0, 6, 12, and 24 h and lysed in RIPA-Doc buffer containing a series of serine and tyrosine phosphatase inhibitors. The cell lysates were subjected to Western blot analysis using a rabbit anti–phospho-GSK-3βSer-9 antibody. Kodak Image Analysis software was used to quantify protein level. n = 3. *, P < 0.001 by ANOVA. (F) Total GSK-3β level was also measured. (G) Promoter assay. pTOPFLASH plasmid was cotransfected with pcDNA3-β-catenin and pcDNA3-Tcf expression plasmids into N2a cells. pCMV-Rluc was also cotransfected to normalize transfection efficiency. Luciferase assay was performed 48 h after transfection. Promoter activity was indicated by the luciferase activity. VPA increased β-catenin-Tcf mediated transcription activation. n = 4. *, P < 0.005 by Student's t test.
Similar articles
- Valproic Acid Modifies Synaptic Structure and Accelerates Neurite Outgrowth Via the Glycogen Synthase Kinase-3β Signaling Pathway in an Alzheimer's Disease Model.
Long ZM, Zhao L, Jiang R, Wang KJ, Luo SF, Zheng M, Li XF, He GQ. Long ZM, et al. CNS Neurosci Ther. 2015 Nov;21(11):887-97. doi: 10.1111/cns.12445. Epub 2015 Sep 19. CNS Neurosci Ther. 2015. PMID: 26385876 Free PMC article. - Cryptotanshinone, a compound from Salvia miltiorrhiza modulates amyloid precursor protein metabolism and attenuates beta-amyloid deposition through upregulating alpha-secretase in vivo and in vitro.
Mei Z, Zhang F, Tao L, Zheng W, Cao Y, Wang Z, Tang S, Le K, Chen S, Pi R, Liu P. Mei Z, et al. Neurosci Lett. 2009 Mar 13;452(2):90-5. doi: 10.1016/j.neulet.2009.01.013. Epub 2009 Jan 13. Neurosci Lett. 2009. PMID: 19154776 - Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease.
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Rockenstein E, et al. J Neurosci Res. 2006 May 15;83(7):1252-61. doi: 10.1002/jnr.20818. J Neurosci Res. 2006. PMID: 16511867 - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Roles of glycogen synthase kinase 3 in Alzheimer's disease.
Cai Z, Zhao Y, Zhao B. Cai Z, et al. Curr Alzheimer Res. 2012 Sep;9(7):864-79. doi: 10.2174/156720512802455386. Curr Alzheimer Res. 2012. PMID: 22272620 Review.
Cited by
- "Boomerang Neuropathology" of Late-Onset Alzheimer's Disease is Shrouded in Harmful "BDDS": Breathing, Diet, Drinking, and Sleep During Aging.
Daulatzai MA. Daulatzai MA. Neurotox Res. 2015 Jul;28(1):55-93. doi: 10.1007/s12640-015-9528-x. Epub 2015 Apr 25. Neurotox Res. 2015. PMID: 25911292 Review. - Immunotherapeutic efficiency of a tetravalent Aβ1-15 vaccine in APP/PS1 transgenic mice as mouse model for Alzheimer's disease.
Guan X, Yang J, Gu H, Zou J, Yao Z. Guan X, et al. Hum Vaccin Immunother. 2013 Aug;9(8):1643-53. doi: 10.4161/hv.24830. Epub 2013 May 31. Hum Vaccin Immunother. 2013. PMID: 23732905 Free PMC article. - Epigenetic Regulation of Neuroinflammation in Alzheimer's Disease.
Ma Y, Wang W, Liu S, Qiao X, Xing Y, Zhou Q, Zhang Z. Ma Y, et al. Cells. 2023 Dec 29;13(1):79. doi: 10.3390/cells13010079. Cells. 2023. PMID: 38201283 Free PMC article. Review. - Epigenetic mechanisms in neurological and neurodegenerative diseases.
Landgrave-Gómez J, Mercado-Gómez O, Guevara-Guzmán R. Landgrave-Gómez J, et al. Front Cell Neurosci. 2015 Feb 27;9:58. doi: 10.3389/fncel.2015.00058. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25774124 Free PMC article. Review. - The neurotrophic and neuroprotective effects of psychotropic agents.
Hunsberger J, Austin DR, Henter ID, Chen G. Hunsberger J, et al. Dialogues Clin Neurosci. 2009;11(3):333-48. doi: 10.31887/DCNS.2009.11.3/jhunsberger. Dialogues Clin Neurosci. 2009. PMID: 19877500 Free PMC article. Review.
References
- Glenner, G.G., and C.W. Wong. 1984. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120:885–890. - PubMed
- Sun, X., Y. Wang, H. Qing, M.A. Christensen, Y. Liu, W. Zhou, Y. Tong, C. Xiao, Y. Huang, S. Zhang, et al. 2005. Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J. 19:739–749. - PubMed
- Roberds, S.L., J. Anderson, G. Basi, M.J. Bienkowski, D.G. Branstetter, K.S. Chen, S.B. Freedman, N.L. Frigon, D. Games, K. Hu, et al. 2001. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 10:1317–1324. - PubMed
- Luo, Y., B. Bolon, S. Kahn, B.D. Bennett, S. Babu-Khan, P. Denis, W. Fan, H. Kha, J. Zhang, Y. Gong, et al. 2001. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4:231–232. - PubMed
- Cai, H., Y. Wang, D. McCarthy, H. Wen, D.R. Borchelt, D.L. Price, and P.C. Wong. 2001. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4:233–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous