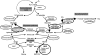The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants - PubMed (original) (raw)
The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants
Marion Eisenhut et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Photorespiratory 2-phosphoglycolate (2PG) metabolism is essential for photosynthesis in higher plants but thought to be superfluous in cyanobacteria because of their ability to concentrate CO(2) internally and thereby inhibit photorespiration. Here, we show that 3 routes for 2PG metabolism are present in the model cyanobacterium Synechocystis sp. strain PCC 6803. In addition to the photorespiratory C2 cycle characterized in plants, this cyanobacterium also possesses the bacterial glycerate pathway and is able to completely decarboxylate glyoxylate via oxalate. A triple mutant with defects in all 3 routes of 2PG metabolism exhibited a high-CO(2)-requiring (HCR) phenotype. All these catabolic routes start with glyoxylate, which can be synthesized by 2 different forms of glycolate dehydrogenase (GlcD). Mutants defective in one or both GlcD proteins accumulated glycolate under high CO(2) level and the double mutant DeltaglcD1/DeltaglcD2 was unable to grow under low CO(2). The HCR phenotype of both the double and the triple mutant could not be attributed to a significantly reduced affinity to CO(2), such as in other cyanobacterial HCR mutants defective in the CO(2)-concentrating mechanism (CCM). These unexpected findings of an HCR phenotype in the presence of an active CCM indicate that 2PG metabolism is essential for the viability of all organisms that perform oxygenic photosynthesis, including cyanobacteria and C3 plants, at ambient CO(2) conditions. These data and phylogenetic analyses suggest cyanobacteria as the evolutionary origin not only of oxygenic photosynthesis but also of an ancient photorespiratory 2PG metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
A scheme displaying the 2PG metabolism in Synechocystis sp. strain PCC 6803, which employs 3 different routes: C2 cycle, glycerate pathway, and the decarboxylating branch. Enzymatic steps mutated in strains used for this study are indicated in bold.
Fig. 2.
Genotypic and phenotypic characterization of the triple mutant Δ_gcvT_/Δ_tsr_/Δ_odc_. (A) Complete segregation of the mutant Δ_gcvT_/Δ_tsr_/Δ_odc_ was verified by PCR with gene-specific primers (see
SI Table 1
). (B) Growth of WT, single mutant Δ_odc_ blocked in the decarboxylation branch, double mutant Δ_odc_/Δ_gcvT_ blocked in the decarboxylation branch and C2 cycle, and triple mutant Δ_gcvT_/Δ_tsr_/Δ_odc_ blocked in all 3 branches of 2PG metabolism (see Fig. 1), respectively, under HC or LC. Strains were plated on BG11, pH 7, solidified by 0.9% Kobe agar, and incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C for 7 d. (C) Resistance of WT, single mutant Δ_odc_, double mutant Δ_odc_/Δ_gcvT_, and triple mutant Δ_gcvT_/Δ_tsr_/Δ_odc_ toward oxalate (Ox). Strains were plated on BG11 agar plates, pH 8, supplemented by different amounts of oxalate. Cells were incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C and HC for 7 d. [C reproduced with permission from ref. 41].
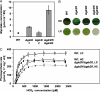
Fig. 3.
Phenotypic characterization of single mutants and a double mutant of Synechocystis defective in GlcDs. (A) Quantification of intracellular glycolate in cells of single- (Δ_glcD1_, Δ_glcD2_) and double- (Δ_glcD1_/Δ_glcD2_) mutants in the glycolate converting step. Samples were taken 3 h after shift from HC to LC and glycolate was quantified by HPLC. *, WT cells contained only traces of glycolate under these conditions. (B) Growth of WT, single mutants Δ_glcD1_ or Δ_glcD2_, and double mutant Δ_glcD1_/Δ_glcD2_ under HC or LC. Strains were plated on BG11, pH 7, solidified by 0.9% Kobe agar, and incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C for 7 d. (C) Photosynthesis rates of cells of the WT and the double mutant Δ_glcD1_/Δ_glcD2_ at different concentrations of HCO3− as a source for Ci. The cells were grown in liquid BG11 medium at HC or transferred to aeration by ambient air (LC) for 6 h.
Similar articles
- The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria.
Eisenhut M, Kahlon S, Hasse D, Ewald R, Lieman-Hurwitz J, Ogawa T, Ruth W, Bauwe H, Kaplan A, Hagemann M. Eisenhut M, et al. Plant Physiol. 2006 Sep;142(1):333-42. doi: 10.1104/pp.106.082982. Epub 2006 Jul 28. Plant Physiol. 2006. PMID: 16877700 Free PMC article. - Pathway and importance of photorespiratory 2-phosphoglycolate metabolism in cyanobacteria.
Hagemann M, Eisenhut M, Hackenberg C, Bauwe H. Hagemann M, et al. Adv Exp Med Biol. 2010;675:91-108. doi: 10.1007/978-1-4419-1528-3_6. Adv Exp Med Biol. 2010. PMID: 20532737 - Low-carbon acclimation in carboxysome-less and photorespiratory mutants of the cyanobacterium Synechocystis sp. strain PCC 6803.
Hackenberg C, Huege J, Engelhardt A, Wittink F, Laue M, Matthijs HCP, Kopka J, Bauwe H, Hagemann M. Hackenberg C, et al. Microbiology (Reading). 2012 Feb;158(Pt 2):398-413. doi: 10.1099/mic.0.054544-0. Epub 2011 Nov 17. Microbiology (Reading). 2012. PMID: 22096149 - Evolution of photorespiration from cyanobacteria to land plants, considering protein phylogenies and acquisition of carbon concentrating mechanisms.
Hagemann M, Kern R, Maurino VG, Hanson DT, Weber AP, Sage RF, Bauwe H. Hagemann M, et al. J Exp Bot. 2016 May;67(10):2963-76. doi: 10.1093/jxb/erw063. Epub 2016 Mar 1. J Exp Bot. 2016. PMID: 26931168 Review. - Photorespiratory glycolate-glyoxylate metabolism.
Dellero Y, Jossier M, Schmitz J, Maurino VG, Hodges M. Dellero Y, et al. J Exp Bot. 2016 May;67(10):3041-52. doi: 10.1093/jxb/erw090. Epub 2016 Mar 19. J Exp Bot. 2016. PMID: 26994478 Review.
Cited by
- Comparative transcriptome profiling and weighted gene co-expression network analysis to identify core genes in maize (Zea mays L.) silks infected by multiple fungi.
Kumar A, Kanak KR, Arunachalam A, Dass RS, Lakshmi PTV. Kumar A, et al. Front Plant Sci. 2022 Oct 27;13:985396. doi: 10.3389/fpls.2022.985396. eCollection 2022. Front Plant Sci. 2022. PMID: 36388593 Free PMC article. - Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure.
Ishaq SL, Johnson SP, Miller ZJ, Lehnhoff EA, Olivo S, Yeoman CJ, Menalled FD. Ishaq SL, et al. Microb Ecol. 2017 Feb;73(2):417-434. doi: 10.1007/s00248-016-0861-2. Epub 2016 Sep 27. Microb Ecol. 2017. PMID: 27677892 - Expression profiling of the bloom-forming cyanobacterium Nodularia CCY9414 under light and oxidative stress conditions.
Kopf M, Möke F, Bauwe H, Hess WR, Hagemann M. Kopf M, et al. ISME J. 2015 Oct;9(10):2139-52. doi: 10.1038/ismej.2015.16. Epub 2015 Feb 17. ISME J. 2015. PMID: 25689027 Free PMC article. - Efficient 2-phosphoglycolate degradation is required to maintain carbon assimilation and allocation in the C4 plant Flaveria bidentis.
Levey M, Timm S, Mettler-Altmann T, Luca Borghi G, Koczor M, Arrivault S, Pm Weber A, Bauwe H, Gowik U, Westhoff P. Levey M, et al. J Exp Bot. 2019 Jan 7;70(2):575-587. doi: 10.1093/jxb/ery370. J Exp Bot. 2019. PMID: 30357386 Free PMC article. - Cyanobacterial lactate oxidases serve as essential partners in N2 fixation and evolved into photorespiratory glycolate oxidases in plants.
Hackenberg C, Kern R, Hüge J, Stal LJ, Tsuji Y, Kopka J, Shiraiwa Y, Bauwe H, Hagemann M. Hackenberg C, et al. Plant Cell. 2011 Aug;23(8):2978-90. doi: 10.1105/tpc.111.088070. Epub 2011 Aug 9. Plant Cell. 2011. PMID: 21828292 Free PMC article.
References
- Tolbert NE. The C-2 oxidative photosynthetic carbon cycle. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:1–25. - PubMed
- Deusch O, et al. Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor. Mol Biol Evol. 2008;25:748–761. - PubMed
- Husic DW, Husic HD, Tolbert NE. The oxidative photosynthetic carbon cycle or C2 cycle. CRC Crit Rev Plant Sci. 1987;5:45–100.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous