T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS - PubMed (original) (raw)
T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS
Isaac M Chiu et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset, progressive, motor neuron degenerative disease, in which the role of inflammation is not well established. Innate and adaptive immunity were investigated in the CNS of the Superoxide Dismutase 1 (SOD1)(G93A) transgenic mouse model of ALS. CD4+ and CD8+ T cells infiltrated SOD1(G93A) spinal cords during disease progression. Cell-specific flow cytometry and gene expression profiling showed significant phenotypic changes in microglia, including dendritic cell receptor acquisition, and expression of genes linked to neuroprotection, cholesterol metabolism and tissue remodeling. Microglia dramatically up-regulated IGF-1 and down-regulated IL-6 expression. When mutant SOD1 mice were bred onto a TCRbeta deficient background, disease progression was significantly accelerated at the symptomatic stage. In addition, microglia reactivity and IGF-1 levels were reduced in spinal cords of SOD1(G93A) (TCRbeta-/-) mice. These results indicate that T cells play an endogenous neuroprotective role in ALS by modulating a beneficial inflammatory response to neuronal injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Microglia and lymphocyte dynamics in spinal cords of ALS Tg mice. (A) Spinal cords analyzed for resident microglia, infiltrating lymphocytes, monocytes at day 65, day 100, and day 135; representative populations are labeled (A), (B), and (C). (B–C) Cumulative analyses of lymphocytes. Resident microglia show specific increases in SOD1G93A spinal cord over time (_p_-values by t test). (D) Representative lymphocyte analysis for CD4+, CD8+ T cells, CD19+ B cells, and NK1.1+ natural killer cells. (E) Proportional increases in specific lymphocyte subsets relative to total immune cells in spinal cord, brain of SOD1G93A mice. (Error bars show SEM.)
Fig. 2.
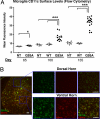
Microglia acquire dendritic cell markers in ventral horns of SOD1G93A Tg mice. (A) Spinal cord microglia were analyzed for CD11c surface levels by FACS. SOD1G93A(G93A), but not non-Tg(NT) or SOD1WT(WT) microglia show increases in CD11c MFI (One-way ANOVA, *, P < 0.05; ***, P < 0.001) (B) CD11b+ microglia expressing CD11c accumulate in ventral horns, but not dorsal horns, of SOD1G93A spinal cord. Lumbar section at day 135 with corresponding high magnification images are shown. (Scale bar, 50 μm.)
Fig. 3.
Expression profile of directly purified microglia in mutant SOD1 mice. Microglia were purified by density gradient, magnetic bead selection from spinal cords of SOD1G93A, SOD1WT and non-Tg mice. Expression levels of (A) IGF-1 (B) Osteopontin (C) IL-1R antagonist and (D) IL-6 were determined by quantitative PCR. Statistical analysis between SOD1G93A and non-Tg microglia are by t test.
Fig. 4.
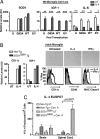
The Th2 Cytokine IL-4, a factor in modulation of microglia response. (A) N9 microglia cells, uninfected (U) or transduced with human SOD1G93A (G93A), SOD1WT (WT), empty vector (EV) lentivirus, were treated with IL-4, and assayed for human SOD1, IGF-1, IL-6 expression. Experiments performed in triplicate, *, P < 0.05; **, P < 0.01; and ***, P < 0.001, by t test. (B) Primary adult microglia cultured from SOD1G93A or non-Tg mice were treated with IL-4 or IFN-γ. IL-4 induced IGF-1 and CD11c expression by quantitative PCR. Microglia display morphological changes by light microscopy. By FACS, IL-4 induces CD11c while IFN-γ induces MHC Class II surface expression; CD40 levels were unaffected (tinted histograms, untreated microglia) (C) IL-4 ELISPOT performed on CNS isolated leukocytes at day 135, stimulated with Con A (Con. A) or untreated (UT) for 48 h; numbers of IL-4 secreting cell colonies were determined (P value by t test).
Fig. 5.
Decreased microglia reactivity and IGF-1 expression in absence of T cells. (A) Spinal cord lumbar sections from non-Tg, SOD1G93A and SOD1G93A TCR−/− end-stage mice were stained for CD68 (microglia, red), GFAP (astrocytes, green) and imaged by confocal microscopy. In the absence of T cells, CD68 fluorescence decreases while GFAP is unchanged. (Scale bar, 20 μm.) Cumulative analysis of CD68, and GFAP pixels in serial sections (n.s., not significant) (B) Lumbar sections were stained for IGF-1 (red) and CD11b (green). The CD11b antibody used (M1/70) only recognized activated microglia in fixed tissue sections. In SOD1G93A spinal cord, microglia co-localized with expression of IGF-1 (60x magnification), and in SOD1G93A TCR−/− mice, CD11b and IGF-1 staining decreased (Ventral horn gray matter is delineated by dotted white lines). Scale bar = 50 μm. Image analysis of IGF-1 integrated intensity/CD11b (C), IGF-1 (I), and co-localized (Col) pixels show the dependence of IGF-1 levels on T cells (one way ANOVA, ***, P < 0.001.)
Fig. 6.
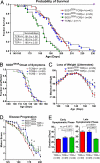
Accelerated disease progression in mutant SOD1 mice deficient in T cells. (A–B) Kaplan Meier curves for survival and symptomatic onset in SOD1G93A (males, n = 22; females, n = 20), SOD1G93ATCR± (m, n = 16; f, n = 13), SOD1G93ATCR−/− (m, n = 13; f, n = 11) and TCR−/− (m, n = 7; f, n = 10) animals. (C) Weight loss plotted for SOD1G93ATCR± and TCR−/− littermates. (D) Decline in motor symptoms scored over time between genotypes. For C and D, statistically significant time-points are indicated; *, P < 0.05 by t test. (E) Duration of early and late symptomatic phases of disease for SOD1G93ATCR+/+, TCR±, and TCR−/− mice. Statistical analysis by one-way ANOVA.
Similar articles
- Effect of thymic stimulation of CD4+ T cell expansion on disease onset and progression in mutant SOD1 mice.
Sheean RK, Weston RH, Perera ND, D'Amico A, Nutt SL, Turner BJ. Sheean RK, et al. J Neuroinflammation. 2015 Feb 27;12:40. doi: 10.1186/s12974-015-0254-3. J Neuroinflammation. 2015. PMID: 25889790 Free PMC article. - Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase.
Gowing G, Philips T, Van Wijmeersch B, Audet JN, Dewil M, Van Den Bosch L, Billiau AD, Robberecht W, Julien JP. Gowing G, et al. J Neurosci. 2008 Oct 8;28(41):10234-44. doi: 10.1523/JNEUROSCI.3494-08.2008. J Neurosci. 2008. PMID: 18842883 Free PMC article. - Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.
Fellner A, Barhum Y, Angel A, Perets N, Steiner I, Offen D, Lev N. Fellner A, et al. Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article. - Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis.
Rizzo F, Riboldi G, Salani S, Nizzardo M, Simone C, Corti S, Hedlund E. Rizzo F, et al. Cell Mol Life Sci. 2014 Mar;71(6):999-1015. doi: 10.1007/s00018-013-1480-4. Epub 2013 Oct 8. Cell Mol Life Sci. 2014. PMID: 24100629 Free PMC article. Review. - Microglia in ALS: the good, the bad, and the resting.
Henkel JS, Beers DR, Zhao W, Appel SH. Henkel JS, et al. J Neuroimmune Pharmacol. 2009 Dec;4(4):389-98. doi: 10.1007/s11481-009-9171-5. J Neuroimmune Pharmacol. 2009. PMID: 19731042 Review.
Cited by
- The role of macrophage plasticity in neurodegenerative diseases.
Ma H, Zhu M, Chen M, Li X, Feng X. Ma H, et al. Biomark Res. 2024 Aug 13;12(1):81. doi: 10.1186/s40364-024-00624-7. Biomark Res. 2024. PMID: 39135084 Free PMC article. Review. - Association of serum Spp1 levels with disease progression in ALS and SBMA.
Ju W, Ban JJ, Im HR, Ko SH, Seo J, Min YG, Hong YH, Choi SJ, Sung JJ. Ju W, et al. Ann Clin Transl Neurol. 2024 Jul;11(7):1809-1818. doi: 10.1002/acn3.52087. Epub 2024 May 22. Ann Clin Transl Neurol. 2024. PMID: 38775192 Free PMC article. - Circulating Levels of T-Cell Traits and the Risk of Amyotrophic Lateral Sclerosis: A Mendelian Randomization Study.
Lu T, Luo L, Yang J, Cheng X, Sun J. Lu T, et al. Mol Neurobiol. 2024 May 15. doi: 10.1007/s12035-024-04226-0. Online ahead of print. Mol Neurobiol. 2024. PMID: 38748065 - Targeting low levels of MIF expression as a potential therapeutic strategy for ALS.
Alfahel L, Gschwendtberger T, Kozareva V, Dumas L, Gibbs R, Kertser A, Baruch K, Zaccai S, Kahn J, Thau-Habermann N, Eggenschwiler R, Sterneckert J, Hermann A, Sundararaman N, Vaibhav V, Van Eyk JE, Rafuse VF, Fraenkel E, Cantz T, Petri S, Israelson A. Alfahel L, et al. Cell Rep Med. 2024 May 21;5(5):101546. doi: 10.1016/j.xcrm.2024.101546. Epub 2024 May 3. Cell Rep Med. 2024. PMID: 38703766 Free PMC article. - CNS autoimmune response in the MAM/pilocarpine rat model of epileptogenic cortical malformation.
Costanza M, Ciotti A, Consonni A, Cipelletti B, Cattalini A, Cagnoli C, Baggi F, de Curtis M, Colciaghi F. Costanza M, et al. Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2319607121. doi: 10.1073/pnas.2319607121. Epub 2024 Apr 18. Proc Natl Acad Sci U S A. 2024. PMID: 38635635 Free PMC article.
References
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature reviews. 2006;7:710–723. - PubMed
- Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle and Nerve. 2002;26:459–470. - PubMed
- Clement AM, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. - PubMed
- Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous