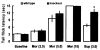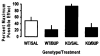Buprenorphine: a unique drug with complex pharmacology - PubMed (original) (raw)
Buprenorphine: a unique drug with complex pharmacology
Kabirullah Lutfy et al. Curr Neuropharmacol. 2004 Oct.
Abstract
Buprenorphine, an opioid with mixed agonist-antagonist activity at classical opioid receptors, has been approved recently for the treatment of opioid dependency. Buprenorphine is also used as an analgesic. The buprenorphine dose-response curve is sometimes submaximal, or even bell-shaped, in nociceptive assays, depending upon the nature and intensity of the noxious stimulus. Moreover, buprenorphine, when administered with full agonists, such as morphine, antagonizes the action of these drugs. Partial agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptor have been considered as possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. While ceiling effects can be explained by partial agonist activity of buprenorphine, the bell-shaped dose-response curve cannot be a consequence of this property of the drug. Recently, buprenorphine has been shown to activate the opioid receptor-like (ORL-1; also known as NOP) receptor. Supraspinal activation of the ORL-1 receptor counteracts the antinociceptive and rewarding actions of morphine, raising the possibility that these actions of buprenorphine can also be altered by its ability to concomitantly activate the ORL-1 receptor. The use of molecular biological techniques has advanced our knowledge regarding the role of opioid receptors in modulation of pain and reward. In particular, generation of opioid receptor knockout mice has proven useful in this regard. Indeed, using knockout mice, we have recently shown that the antinociceptive effect of buprenorphine mediated primarily by the mu opioid receptor is attenuated by the ability of the drug to activate the ORL-1 receptor. Thus, the goal of this review is to provide evidence demonstrating that the ORL-1 receptor plays a functional role not only in the antinociceptive effect of buprenorphine but also in other actions of the drug as well.
Keywords: Buprenorphine; ORL-1 receptors; agonist-antagonist; antinociception; dependence; knockout mice; partial agonist; tolerance.
Figures
Fig. (1)
Buprenorphine attenuates morphine-induced antinociception in wild type, but not ORL-1 receptor knockout, mice in the radiant heat tail flick assay. Mice were tested for baseline tail flick latency, injected with morphine (2.5 mg/kg, s.c.) and tested 30 min later. The same mice were injected with the next dose of morphine (5 mg/kg,) and tested 30 min later. After the tail flick latency was measured 30 min following the last dose of morphine (10 mg/kg, s.c.), mice were injected with buprenorphine (3 mg/kg, s.c.) and tested after a further 15-min delay. Data are means (± s.e.m.) of 5–6 mice/genotype. *indicates a significant difference between wild type (WT) and knockout (KO) mice (t = 4.07; p<0.05).
Fig. (2)
Tolerance develops to the antinociceptive effect of buprenorphine in mice lacking the ORL-1 receptor in the radiant heat tail flick assay. Mice were injected with either saline or buprenorphine (1 mg/kg, s.c.) once daily for 4 days. On day 5, mice were tested for baseline tail flick latency, injected with buprenorphine (1 mg/kg, s.c.) and re-tested after 15 min. Percent maximum possible effect (%MPE) was obtained using the formula: %MPE = [(Test latency − Baseline) / (Cut-off (15 sec) − Baseline)] × 100. Data are means (+ s.e.m.) of 5–7 mice/treatment/group.
Similar articles
- Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors.
Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Lutfy K, et al. J Neurosci. 2003 Nov 12;23(32):10331-7. doi: 10.1523/JNEUROSCI.23-32-10331.2003. J Neurosci. 2003. PMID: 14614092 Free PMC article. - Buprenorphine-Clinically useful but often misunderstood.
Butler S. Butler S. Scand J Pain. 2013 Jul 1;4(3):148-152. doi: 10.1016/j.sjpain.2013.05.004. Scand J Pain. 2013. PMID: 29913911 - Buprenorphine antinociception is abolished, but naloxone-sensitive reward is retained, in mu-opioid receptor knockout mice.
Ide S, Minami M, Satoh M, Uhl GR, Sora I, Ikeda K. Ide S, et al. Neuropsychopharmacology. 2004 Sep;29(9):1656-63. doi: 10.1038/sj.npp.1300463. Neuropsychopharmacology. 2004. PMID: 15100703 - Buprenorphine: new pharmacological aspects.
Cowan A. Cowan A. Int J Clin Pract Suppl. 2003 Feb;(133):3-8; discussion 23-4. Int J Clin Pract Suppl. 2003. PMID: 12665117 Review. - Mixed κ/μ partial opioid agonists as potential treatments for cocaine dependence.
Bidlack JM. Bidlack JM. Adv Pharmacol. 2014;69:387-418. doi: 10.1016/B978-0-12-420118-7.00010-X. Adv Pharmacol. 2014. PMID: 24484983 Review.
Cited by
- Buprenorphine reduces methamphetamine intake and drug seeking behavior via activating nociceptin/orphanin FQ peptide receptor in rats.
Wang F, Shen W, Cai Y, Zhang X, Du H, Lai M, Liu H, Kohli E, Zhou W. Wang F, et al. Front Psychiatry. 2022 Oct 6;13:983595. doi: 10.3389/fpsyt.2022.983595. eCollection 2022. Front Psychiatry. 2022. PMID: 36276332 Free PMC article. - Oxycodone, an opioid like the others?
Marie N, Noble F. Marie N, et al. Front Psychiatry. 2023 Dec 13;14:1229439. doi: 10.3389/fpsyt.2023.1229439. eCollection 2023. Front Psychiatry. 2023. PMID: 38152360 Free PMC article. Review. - Buprenorphine as a Treatment for Major Depression and Opioid Use Disorder.
Namchuk AB, Lucki I, Browne CA. Namchuk AB, et al. Adv Drug Alcohol Res. 2022;2:10254. doi: 10.3389/adar.2022.10254. Epub 2022 Feb 21. Adv Drug Alcohol Res. 2022. PMID: 36177442 Free PMC article. - Sublingual buprenorphine/naloxone for chronic pain in at-risk patients: development and pilot test of a clinical protocol.
Rosenblum A, Cruciani RA, Strain EC, Cleland CM, Joseph H, Magura S, Marsch LA, McNicholas LF, Savage SR, Sundaram A, Portenoy RK. Rosenblum A, et al. J Opioid Manag. 2012 Nov-Dec;8(6):369-82. doi: 10.5055/jom.2012.0137. J Opioid Manag. 2012. PMID: 23264315 Free PMC article. - A role for the mu opioid receptor in the antidepressant effects of buprenorphine.
Robinson SA, Erickson RL, Browne CA, Lucki I. Robinson SA, et al. Behav Brain Res. 2017 Feb 15;319:96-103. doi: 10.1016/j.bbr.2016.10.050. Epub 2016 Nov 3. Behav Brain Res. 2017. PMID: 27818236 Free PMC article.
References
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization) to the excitatory effects of morphine. A motility study in post-depentent rats. Neuropharmacology. 1983;22:1193–1196. - PubMed
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the Opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. - PubMed
- Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. Behavioral and neurochemical interactions between cocaine and buprenorphine: implications for the pharmacotheraphy of cocaine abuse. J Pharmacol Exp Ther. 1991;256:119–126. - PubMed
- Budd K, Collett BJ. Old dog--new (ma)trix. Br J Anaesth. 2003;90:722–724. - PubMed
- Bunzow JR, Snez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials