Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases - PubMed (original) (raw)
Review
Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases
Peter J Adhihetty et al. Neuromolecular Med. 2008.
Abstract
Substantial evidence indicates bioenergetic dysfunction and mitochondrial impairment contribute either directly and/or indirectly to the pathogenesis of numerous neurodegenerative disorders. Treatment paradigms aimed at ameliorating this cellular energy deficit and/or improving mitochondrial function in these neurodegenerative disorders may prove to be useful as a therapeutic intervention. Creatine is a molecule that is produced both endogenously, and acquired exogenously through diet, and is an extremely important molecule that participates in buffering intracellular energy stores. Once creatine is transported into cells, creatine kinase catalyzes the reversible transphosphorylation of creatine via ATP to enhance the phosphocreatine energy pool. Creatine kinase enzymes are located at strategic intracellular sites to couple areas of high energy expenditure to the efficient regeneration of ATP. Thus, the creatine kinase/phosphocreatine system plays an integral role in energy buffering and overall cellular bioenergetics. Originally, exogenous creatine supplementation was widely used only as an ergogenic aid to increase the phosphocreatine pool within muscle to bolster athletic performance. However, the potential therapeutic value of creatine supplementation has recently been investigated with respect to various neurodegenerative disorders that have been associated with bioenergetic deficits as playing a role in disease etiology and/or progression which include; Alzheimer's, Parkinson's, amyotrophic lateral sclerosis (ALS), and Huntington's disease. This review discusses the contribution of mitochondria and bioenergetics to the progression of these neurodegenerative diseases and investigates the potential neuroprotective value of creatine supplementation in each of these neurological diseases. In summary, current literature suggests that exogenous creatine supplementation is most efficacious as a treatment paradigm in Huntington's and Parkinson's disease but appears to be less effective for ALS and Alzheimer's disease.
Figures
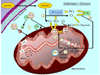
Fig. 1
Creatine kinase/phosphocreatine energy shuttle pathway and the role of mitochondria in apoptotic cell death in neurons. (1) Mitochondrial involvement in apoptosis. Mitochondria are intimately involved in apoptotic cell death since, (1) they contain pro-apoptotic proteins which can lead to cell death upon release into the cytosol (DNA fragmentation is a hallmark feature of apoptosis), and (2) they produce the majority of the potentially damaging reactive oxygen species (ROS). ROS can damage intracellular components (i.e., alter DNA, proteins, lipids) and have also been shown to increase the susceptibility to apoptosis by modulating components of the channel that traverses the inner and outer mitochondrial membrane termed the mitochondrial permeability transition pore (mtPTP). Upon a significant apoptotic insult, mitochondria can release pro-apoptotic factors to the cytosol to induce cellular death. The octameric form of the mitochondrial creatine kinase (MtCK) interacts with components of the mtPTP to suppress pore opening and potentially reduce apoptotic susceptibility. Creatine has been shown to maintain the mtCK in an octameric conformation (as opposed to dimeric) which inhibits mtPTP opening. (2) Creatine kinase/phosphocreatine energy shuttle pathway. Creatine (Cr) is taken up by the neuron via specific creatine transporters and transphosphorylated to the high-energy phosphocreatine (PCr) by either mitochondrial creatine kinases (MtCKs) or cytosolic creatine kinases (CKs). MtCK is located in the intermembrane space of mitochondria and is coupled to ATP production by mitochondrial oxidative phosphorylation to produce PCr which is then exported from the organelle. Cytosolic CK transphosphorylates ATP generated via glycolysis to PCr. This PCr can then contribute to the overall cellular PCr pool. The PCr pool is coupled to localized CKs in areas of high ATP demand such as (2a) organelle transport and/or ATP dependent cell signaling or (2b) at the cell membrane by ATP-dependent pumps and/or ATP-dependent receptors. This localized distribution of CKs allows for the efficient production of ATP from ADP to fuel ATP-dependent processes in various portions of the cell. (3) Effect of exogenous creatine supplementation (dashed lines). Exogenous creatine is transported through the cell membrane via creatine transporters to increase the overall creatine pool. (3a) Increased creatine levels may further stabilize the octameric form of mtCK to suppress opening of the mtPTP and this may reduce mitochondrially mediated apoptosis. (3b) Elevated levels of creatine within the cell can be transphosphorylated by mitochondrial and cytosolic creatine kinases (MtCKs and CKs, respectively) to enhance the overall PCr pool. Increased PCr levels can be utilized by localized creatine kinases to transphosphorylate ADP to ATP for various ATP-dependent processes within the cell (2a) and at the cell membrane (2b). (4) Potential neuroprotective effects of creatine. Creatine supplementation may improve both the bioenergetic and mitochondrial deficits associated with some neurological diseases (more efficacious in Parkinson’s and Huntington’s disease). These beneficial effects of creatine may ultimately reduce neuronal cell death and provide neuroprotection in certain neurodegenerative disorders
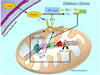
Fig. 2
Mitochondrial involvement in Alzheimer’s disease (AD) and the effect of creatine supplementation. (1) Mitochondrial ROS-induced damage. AD is associated with a greater level of mitochondrially mediated oxidative damage to intracellular proteins and lipids, and this elevated oxidative stress can induce damage to mitochondrial DNA. (2) ROS-induced inactivation of mitochondrial and cytosolic creatine kinase. (2a) Elevated ROS can evoke an octameric-to-dimeric conformational shift in mitochondrial creatine kinase (MtCK-oct) leading to an inactive form of the enzyme (MtCK-dim). (2b) Elevated ROS can also cause damage to, and reduce the activity of the cytosolic creatine kinase (CK). The decreased activity of both the mitochondrial and cytosolic creatine kinases results in less generation of phosphocreatine. This leads to an excess accumulation of creatine and the formation of creatine deposits in the cytosol. (3) Cleavage of amyloid precursor peptide (APP) to Aβ inhibits chaperone-like activity to reduce mtCK in mitochondria. APP can bind to MtCK and is proposed to have chaperone-like activity that facilitates translocation of MtCK from the cytosol to mitochondria. In AD, APP is cleaved to form Aβ fragments that are incapable of this chaperone-like activity. (4) Exogenous creatine supplementation. Improvements in cellular bioenergetics in AD with exogenous creatine supplementation is dependent on (a) the extent of inactivation of the mitochondrial and cytosolic CK and, (b) the levels of reduced mitochondrial CK due to suppressed translocation to the mitochondria. Given that creatine deposition sites have found in late-stage AD, creatine supplementation would most likely be efficacious only during early stages of AD when mitochondrial and cytosolic CK might be suppressed but still active to be capable of generating and enhancing the PCr pool
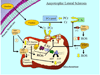
Fig. 3
Mitochondrial involvement in Parkinson’s disease (PD) and the potential therapeutic efficacy of creatine supplementation. (1) PD is characterized by impaired activity of complex I in the ETC of mitochondria. In support of a mitochondrially based etiology in PD, administration of the mitochondrial-specific neurotoxin, MPTP, in animal models, inhibits complex I of the ETC and leads to PD-like pathogenesis. Suppression and/or inhibition of complex I of the ETC lowers overall mitochondrial ATP production and consequently diminishes the ATP (dashed lines) that can be potentially utilized for PCr generation by the MtCK. 2) Additionally, impaired ETC function increases ROS production to induce an octameric-to-dimeric shift of MtCK rendering it an inactive state and elevated ROS will also reduce cytosolic CK activity. (3) Data have shown that creatine supplementation is an efficacious treatment paradigm for PD animal models, but the exact molecular mechanisms are not fully elucidated. However, exogenous creatine appears to improve overall cellular bioenergetics and mitochondrial function by enhancing the PCr pool and this reduces the neuronal cell loss associated with PD pathogenesis. Given the promising results of creatine in animal models of PD, a phase III clinical study of creatine supplementation in PD patients is now underway
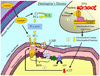
Fig. 4
Schematic illustrating the relationship of mitochondria to amyotrophic lateral sclerosis (ALS) and the potential benefit of creatine supplementation. The most common form of familial ALS (FALS) is caused by numerous different mutations in the metallo-proteinase, superoxide dismutase I (SODI). SODI is an antioxidant that is ubiquitously expressed in the cytosolic fraction of cells and catalyzes the dismutation of superoxide (a highly reactive free radical) to the less reactive hydrogen peroxide (H2O2) and water. (1) Elevations in ROS may occur due to mutations in SOD1, causing reduced SOD1 antioxidant activity. Although somewhat counterintuitive, data have shown that this does not play a major role in the pathogenesis associated with SOD1 mutations. (2) Alternatively, the effects of mutant SOD1 are due to toxic gain-of-function properties. While SOD1 is primarily located in the cytosolic portion of the cell, mutant SOD1 has been found in the intermembrane space and the matrix of mitochondria. Although the detailed molecular mechanisms are not completely understood, mitochondrial mutant SOD1 is associated with impaired ETC function, reduced mitochondrial membrane potential, elevated mitochondrial ROS production, mitochondrial swelling, and intra-mitochondrial vacuole formation. These alterations likely contribute to reduced ATP generation by mitochondria and creatine supplementation in ALS may serve to buffer these mutant SOD1-induced impairments in intracellular bioenergetics. Creatine supplementation was shown to be neuroprotective in animal models of ALS (G93A), but despite promise, was ineffective in clinical trials with ALS patients
Fig. 5
Mitochondrially associated mechanisms involved in Huntington’s disease and the potential effect of creatine supplementation. Mutant huntingtin has been proposed to confer toxic effects to neural tissue by a variety of different mechanisms. (1) With respect to mitochondrial dysfunction, mutant huntingtin has been shown to impair the levels of an important cofactor, PGC-1α, which is involved in regulating the expression nuclear genes encoding mitochondrial proteins (NUGEMPs). (2) Reductions in NUGEMPs can potentially lead to a decrease in the overall content and function in mitochondria. (3) As a consequence, these changes can lead to a reduction in ATP levels and enhanced ROS production. (4) Evidence has also shown that ETC complex activities are impaired in postmortem HD brain tissue. Additionally, in animal models, administration of the neurotoxin, 3-NP, inhibits complex II of the ETC, and leads to Huntington’s-like pathogenesis which underscores the importance of mitochondrial dysfunction in HD. Creatine supplementation reduces lesion volume in the mitochondrial toxin (3-NP) models of HD and provides significant neuroprotection. Given the promising neuroprotective effects of creatine found in animal and phase II clinical studies, a phase III clinical trial in HD patients is currently ongoing
Similar articles
- Functions and effects of creatine in the central nervous system.
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Andres RH, et al. Brain Res Bull. 2008 Jul 1;76(4):329-43. doi: 10.1016/j.brainresbull.2008.02.035. Epub 2008 Mar 24. Brain Res Bull. 2008. PMID: 18502307 Review. - Mitochondrial biogenesis: pharmacological approaches.
Valero T. Valero T. Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795 - The neuroprotective role of creatine.
Klein AM, Ferrante RJ. Klein AM, et al. Subcell Biochem. 2007;46:205-43. doi: 10.1007/978-1-4020-6486-9_11. Subcell Biochem. 2007. PMID: 18652079 Review. - Mitochondrial medicine for aging and neurodegenerative diseases.
Reddy PH. Reddy PH. Neuromolecular Med. 2008;10(4):291-315. doi: 10.1007/s12017-008-8044-z. Epub 2008 Jun 20. Neuromolecular Med. 2008. PMID: 18566920 Free PMC article. Review. - Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.
Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AM, Butterfield DA. Calabrese V, et al. J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
Cited by
- Neuroprotective function of cellular prion protein in a mouse model of amyotrophic lateral sclerosis.
Steinacker P, Hawlik A, Lehnert S, Jahn O, Meier S, Görz E, Braunstein KE, Krzovska M, Schwalenstöcker B, Jesse S, Pröpper C, Böckers T, Ludolph A, Otto M. Steinacker P, et al. Am J Pathol. 2010 Mar;176(3):1409-20. doi: 10.2353/ajpath.2010.090355. Epub 2010 Jan 14. Am J Pathol. 2010. PMID: 20075202 Free PMC article. - Evaluating the feasibility of creatine-weighted CEST MRI in human brain at 7 T using a Z-spectral fitting approach.
Singh A, Debnath A, Cai K, Bagga P, Haris M, Hariharan H, Reddy R. Singh A, et al. NMR Biomed. 2019 Dec;32(12):e4176. doi: 10.1002/nbm.4176. Epub 2019 Oct 14. NMR Biomed. 2019. PMID: 31608510 Free PMC article. - Assessment of chicken thigh meat quality of Ross 308 broiler of animal welfare certified farm.
Kim HJ, Shin DJ, Kim HJ, Cho J, Kwon JS, Kim D, Jung JH, Jang A. Kim HJ, et al. Anim Biosci. 2022 Dec;35(12):1957-1966. doi: 10.5713/ab.22.0044. Epub 2022 Jun 30. Anim Biosci. 2022. PMID: 35798041 Free PMC article. - Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities.
Clemente-Suárez VJ, Redondo-Flórez L, Beltrán-Velasco AI, Ramos-Campo DJ, Belinchón-deMiguel P, Martinez-Guardado I, Dalamitros AA, Yáñez-Sepúlveda R, Martín-Rodríguez A, Tornero-Aguilera JF. Clemente-Suárez VJ, et al. Biomedicines. 2023 Sep 7;11(9):2488. doi: 10.3390/biomedicines11092488. Biomedicines. 2023. PMID: 37760929 Free PMC article. Review. - Severe Hyperhomocysteinemia Decreases Creatine Kinase Activity and Causes Memory Impairment: Neuroprotective Role of Creatine.
Kolling J, Longoni A, Siebert C, Dos Santos TM, Marques EP, Carletti J, Pereira LO, Wyse ATS. Kolling J, et al. Neurotox Res. 2017 Nov;32(4):585-593. doi: 10.1007/s12640-017-9767-0. Epub 2017 Jun 27. Neurotox Res. 2017. PMID: 28656547
References
- Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi:10.1126/science.281.5381.1322. - PubMed
- Adhihetty PJ, Hood DA. Mechanisms of apoptosis in skeletal muscle. Basic and applied myology. 2003;13:171–179.
- Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology. 2003;88:99–107. doi:10.1113/eph8802505. - PubMed
- Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. Journal of Neurochemistry. 2000;74:2520–2527. doi:10.1046/j.1471-4159.2000.0742520.x. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous