Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin - PubMed (original) (raw)
Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin
Min Chen et al. J Biol Chem. 2009.
Abstract
The integrin alpha6beta4 is associated with carcinoma progression by contributing to apoptosis resistance, invasion, and metastasis, due in part to the activation of select transcription factors. To identify genes regulated by the alpha6beta4 integrin, we compared gene expression profiles of MDA-MB-435 cells that stably express integrin alpha6beta4 (MDA/beta4) and vector-only-transfected cells (MDA/mock) using Affymetrix GeneChip analysis. Our results show that integrin alpha6beta4 altered the expression of 538 genes (p < 0.01). Of these genes, 36 are associated with pathways implicated in cell motility and metastasis, including S100A4/metastasin. S100A4 expression correlated well with integrin alpha6beta4 expression in established cell lines. Suppression of S100A4 by small interference RNA resulted in a reduced capacity of alpha6beta4-expressing cells to invade a reconstituted basement membrane in response to lysophosphatidic acid. Using small interference RNA, promoter analysis, and chromatin immunoprecipitation, we demonstrate that S100A4 is regulated by NFAT5, thus identifying the first target of NFAT5 in cancer. In addition, several genes that are known to be regulated by DNA methylation were up-regulated dramatically by integrin alpha6beta4 expression, including S100A4, FST, PDLIM4, CAPG, and Nkx2.2. Notably, inhibition of DNA methyltransferases stimulated expression of these genes in cells lacking the alpha6beta4 integrin, whereas demethylase inhibitors suppressed expression in alpha6beta4 integrin-expressing cells. Alterations in DNA methylation were confirmed by bisulfate sequencing, thus suggesting that integrin alpha6beta4 signaling can lead to the demethylation of select promoters. In summary, our data suggest that integrin alpha6beta4 confers a motile and invasive phenotype to breast carcinoma cells by regulating proinvasive and prometastatic gene expression.
Figures
FIGURE 1.
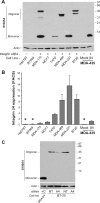
Analysis of MDA-MB-435 clones for select genes altered by α6β4 integrin expression. Total RNA (A) or protein (B) was isolated from the MDA-MB-435 clones 6D2 and 6D7 (MDA/mock; null for the β4 subunit) and 5B3 and 3A7 (MDA/β4; expressing the α6β4 integrin) and submitted for Q-PCR assessment of the indicated genes or immunoblot analysis for the indicated protein, respectively. For extracellular S100A4 (B), conditioned media (CM) represents 50 μl of growth medium removed from cultures just prior to harvesting the cells for protein. For Q-PCR, expression was normalized to 18 S rRNA levels and reported as a value relative to the clone 6D2. Values represent the mean ± S.D.
FIGURE 2.
S100A4 expression correlates with integrin α6β4 expression. A, the indicated breast carcinoma cell lines and MDA-MB-435 clones were harvested at 70% confluence under normal culturing conditions. Cleared whole cell lysates were submitted to SDS-PAGE and immunoblotted for S100A4 (top) or actin (bottom).B, cells were assessed for β4 integrin content by FACS analysis. Data are reported as the average fold difference in mean fluorescence as compared with secondary antibody-only control ± S.D. from three separate experiments. *, cell lines also determined to be negative for β4 integrin expression by immunoblot analysis (data not shown). C, BT-20 cells were electroporated with 200 n
m
siRNA specific for S100A4 (A4) or nontargeting siRNA (NT), as noted, and then cultured for 48 h. Cells were then harvested, and cell lysates were immunoblotted for S100A4. Cell extract from a MDA/β4 transfectant serves as a positive control for the monomeric form (+C).
FIGURE 3.
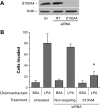
S100A4 is important for chemoinvasion of breast carcinoma cells. MDA-MB-231 cells were electroporated with nothing (Un), nontargeting siRNA (NT), or siRNA targeting S100A4. After 48 h, cells were assessed for S100A4 expression by immunoblot analysis (A) or chemoinvasion toward 100 n
m
LPA (B) as described under “Experimental Procedures.” *, p < 0.002 for treated compared with untreated control and p < 0.0001 for treated compared with nontarget control. BSA, bovine serum albumin.
FIGURE 4.
NFAT5, but not NFAT1, controls the transcriptional regulation of S100A4 in MDA/β4 cells. A and B, MDA/β4 clone 5B3 cells were left untreated (Un) or transfected with either 200 n
m
(1) or 400 n
m
(2) Dharmacon siRNA SMARTPools that are nontargeting (NT) or directed against either NFAT1 (T1) or NFAT5 (T5). After 48 h, cell lysates were harvested and immunoblotted for S100A4, NFAT1, NFAT5, and actin, as indicated (A). Blots from two separate experiments were quantified by densitometry and averaged (B). Bars in B, mean expression ± S.D. *, p value < 0.05 compared with either untreated or nontargeting controls. C and D, MDA/β4 cells were treated with individual siRNAs targeting NFAT5 for 72 or 96 h, and then cell lysates were immunoblotted for S100A4, NFAT5, and actin. E, MDA/mock and MDA/β4 cells under normal culturing conditions were cross-linked with formaldehyde. Nuclei were then isolated, DNA was fragmented, and NFAT5-containing chromatin was immunoprecipitated (IP). The S100A4 promoter associated with NFAT5 was then amplified as described under “Experimental Procedures” and compared with an IgG control.
FIGURE 5.
S100A4 expression is controlled by NFAT5 and integrinα6β4 in MDA-MB-231 cells. A and B, MDA-MB-231 cells were left untreated (Un) or transfected with 200 n
m
siRNA SMARTPools that are nontargeting (NT) or directed against either NFAT1 or NFAT5. Duplicate cells cultures were then harvested 48 h later and analyzed by Q-PCR for S100A4 mRNA expression (A) or protein expression by immunoblot analysis (B). Blot was stripped and reprobed for NFAT1, NFAT5, and actin. For Q-PCR, p values for NFAT5 samples compared with untreated or nontargeting controls were <0.001. C, MDA-MB-231 cells were treated with individual siRNAs targeting NFAT5 for 72 h, and then cell lysates were immunoblotted for S100A4, NFAT5, and actin. D, MDA-MB-231 cells were stably transfected with lentiviral short hairpin RNA constructs that target the integrin β4 subunit (#4 and #5) or that were ineffective in reducing β4 expression (#2). S100A4 expression of these cell populations was compared with the parental cell line by immunoblot analysis. Reduction in integrin β4 expression by short hairpin RNA #4 and #5 was confirmed by FACS analysis (data not shown).
FIGURE 6.
Effect of DNA methyltransferase inhibitor, DAC, and demethylation inhibitor, SAM, on S100A4 expression. A and B, MDA/mock and MDA/β4 transfectants were cultured in the presence or absence of 0.1 or 1 μ
m
DAC for 3 days, as noted. Where indicated, 1 μ
m
tricostatin A (TSA) was added for the final 24 h of culture. Duplicate cultures of each clone were then harvested to assess the level of S100A4 by immunoblot (A) or Q-PCR (B) analysis. Immunoblots in A are from the same gel with the same exposure time. A shorter exposure of the S100A4 blot from a smaller amount of the same samples (1-s exposure) showed that the loading between the MDA/β4 samples was similar. Q-PCR values are reported as -fold change relative to control for each clone. The inset in B represents relative S100A4 level between clones using 3A7 (MDA/β4) control cells as a value of 1.C, MDA-MB-435 clone 5B3 and MDA-MB-231 cells were treated with SAM (80 μ
m
), a methyl donor known to inhibit demethylases, for 3 days under normal culturing conditions prior to harvest and immunoblotting cell lysates for S100A4 and actin. D, genomic DNA from MDA/mock and MDA/β4 transfectants containing the first intron region of the S100A4 promoter (+203 to +662) was assessed for CpG residue methylation by bisulfate conversion and PCR pyrosequencing. The levels of methylation of each of the seven CpG residues in this region are reported. E, the percentages of methylation of CpGs at positions 1, 3, 4, and 5 were averaged and reported as mean ± S.D. TSA, tricostatin A.
FIGURE 7.
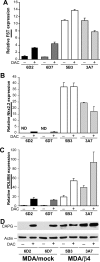
DAC treatment induces expression of several genes in MDA-MB-435 mock transfectants but does not alter expression in MDA/β4 cells. MDA/mock and MDA/β4 transfectants were cultured in the presence of 1 μ
m
DAC for 3 days. Duplicate cultures of each condition were then harvested for RNA to assess the levels of FST (A),Nkx2.2 (B), and PDLIM4 (C) by Q-PCR or protein for CAPG (D). ND in B denotes that message was not detected. Values represent the mean ± S.D.
FIGURE 8.
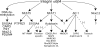
Multilayered regulation of genes downstream of the integrin α6β4. Several transcription factors, including NFAT1, NFAT5, and AP-1 function downstream of the α6β4 integrin. These observations are extended in the current study by identifying genes regulated downstream of NFAT5 (S100A4 and PTPRZ1) and genes regulated by DNA methylation and the α6β4 integrin using the MDA-MB-435 model and using previous analyses of genes regulated by S100A4 (29), autotaxin (34), and AP-1 (35), several of their target genes that are involved in cell motility and invasion that were found regulated by the α6β4 integrin in our gene array analysis.Dashed and solid arrows, negative and positive regulation, respectively. Of note, Cox-2 has been identified as a NFAT1 target; however, this association was not found in our gene array analysis.
Similar articles
- Integrin (alpha6beta4) signals through Src to increase expression of S100A4, a metastasis-promoting factor: implications for cancer cell invasion.
Kim TH, Kim HI, Soung YH, Shaw LA, Chung J. Kim TH, et al. Mol Cancer Res. 2009 Oct;7(10):1605-12. doi: 10.1158/1541-7786.MCR-09-0102. Epub 2009 Oct 6. Mol Cancer Res. 2009. PMID: 19808905 - Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells.
Chen M, O'Connor KL. Chen M, et al. Oncogene. 2005 Jul 28;24(32):5125-30. doi: 10.1038/sj.onc.1208729. Oncogene. 2005. PMID: 15897878 - Src kinase pathway is involved in NFAT5-mediated S100A4 induction by hyperosmotic stress in colon cancer cells.
Chen M, Sastry SK, O'Connor KL. Chen M, et al. Am J Physiol Cell Physiol. 2011 May;300(5):C1155-63. doi: 10.1152/ajpcell.00407.2010. Epub 2011 Feb 2. Am J Physiol Cell Physiol. 2011. PMID: 21289293 - Clinical significance of the integrin α6β4 in human malignancies.
Stewart RL, O'Connor KL. Stewart RL, et al. Lab Invest. 2015 Sep;95(9):976-86. doi: 10.1038/labinvest.2015.82. Epub 2015 Jun 29. Lab Invest. 2015. PMID: 26121317 Free PMC article. Review. - Role of α6β4 integrin in cell motility, invasion and metastasis of mammary tumors.
Soung YH, Gil HJ, Clifford JL, Chung J. Soung YH, et al. Curr Protein Pept Sci. 2011 Feb;12(1):23-9. doi: 10.2174/138920311795659399. Curr Protein Pept Sci. 2011. PMID: 21190525 Review.
Cited by
- S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction.
Dahlmann M, Kobelt D, Walther W, Mudduluru G, Stein U. Dahlmann M, et al. Cancers (Basel). 2016 Jun 20;8(6):59. doi: 10.3390/cancers8060059. Cancers (Basel). 2016. PMID: 27331819 Free PMC article. Review. - Mutations in DNA-binding loop of NFAT5 transcription factor produce unique outcomes on protein-DNA binding and dynamics.
Li M, Shoemaker BA, Thangudu RR, Ferraris JD, Burg MB, Panchenko AR. Li M, et al. J Phys Chem B. 2013 Oct 24;117(42):13226-34. doi: 10.1021/jp403310a. Epub 2013 Jun 25. J Phys Chem B. 2013. PMID: 23734591 Free PMC article. - Effects of β4 integrin expression on microRNA patterns in breast cancer.
Gerson KD, Maddula VS, Seligmann BE, Shearstone JR, Khan A, Mercurio AM. Gerson KD, et al. Biol Open. 2012 Jul 15;1(7):658-66. doi: 10.1242/bio.20121628. Epub 2012 May 25. Biol Open. 2012. PMID: 23213459 Free PMC article. - An efficient expression and purification strategy for the production of S100 proteins in Escherichia coli.
He H, Han L, Guan W, Li J, Han W, Yu Y. He H, et al. Bioengineered. 2013 Jan-Feb;4(1):55-8. doi: 10.4161/bioe.22172. Epub 2012 Sep 18. Bioengineered. 2013. PMID: 22990588 Free PMC article. - Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima.
Liang M, Wang Y, Liang A, Mitch WE, Roy-Chaudhury P, Han G, Cheng J. Liang M, et al. Kidney Int. 2015 Sep;88(3):490-502. doi: 10.1038/ki.2015.73. Epub 2015 Mar 18. Kidney Int. 2015. PMID: 25786100 Free PMC article.
References
- Hynes, R. O. (2002) Cell 110 673–687 - PubMed
- Borradori, L., and Sonnenberg, A. (1999) J. Invest. Dermatol. 112 411–418 - PubMed
- Mercurio, A. M., and Rabinovitz, I. (2001) Semin. Cancer Biol. 11 129–141 - PubMed
- Tagliabue, E., Ghirelli, C., Squicciarini, P., Aiello, P., Colnaghi, M. I., and Menard, S. (1998) Clin. Cancer Res. 4 407–410 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous