Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice - PubMed (original) (raw)
Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice
Smitha Karunakaran et al. J Neurosci. 2008.
Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by the degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNpc). Activation of the mixed lineage kinase and c-Jun N-terminal kinase (JNK) has been reported in models of PD. Our focus was to discern whether distinct pathways were activated in cell-specific manner within the SNpc. We now demonstrate the selective phosphorylation of p38 MAP kinase within the dopaminergic neurons, whereas JNK activation occurs predominantly in the microglia. p38 activation results in downstream phosphorylation of p53 and increased p53 mediated transcription of Bax and Puma in the ventral midbrain. Treatment with p38 inhibitor, SB239063 protected primary dopaminergic neurons derived from human progenitor cells from MPP(+) mediated cell death and prevented the downstream phosphorylation of p53 and its translocation to the nucleus in vivo, in the ventral midbrain. The increased staining of phosphorylated p38 in the surviving neurons of SNpc in human brain sections from patients with PD and in MPTP treated mice but not in the ventral tegmental area provides further evidence suggesting a role for p38 in the degeneration of dopaminergic neurons of SNpc. We thus demonstrate the cell specific activation of MAP kinase pathways within the SNpc after MPTP treatment emphasizing the role of multiple signaling cascades in the pathogenesis and progression of the disease. Selective inhibitors of p38 may therefore, help preserve the surviving neurons in PD and slow down the disease progression.
Figures
Figure 1.
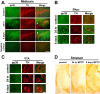
A–D, MPTP-induced activation of p38 and JNK in the ventral midbrain and striatum. Animals were treated with a single dose of vehicle or MPTP and killed 1, 4, 12, and 24 h later or treated with a daily dose of MPTP for 8 d and killed on the ninth day. Representative immunoblots from ventral midbrain (MB) and striatum (ST) of animals treated with saline (C) and MPTP (lanes 1, 4, 12, 24 h, 8 d) depicting (A) the levels of phospho-p38 (pp38) and p38 and (B) JNK54, JNK46, phospho-JNK54 (pJNK54), phospho-JNK46 (pJNK46) in the MB and ST after MPTP treatment. C and D depict the level of JNK54, JNK46, phospho-JNK54 (pJNK54), phospho-JNK46 (pJNK46) in the MB and ST after 8 d of MPTP treatment. β-Tubulin levels were measured as loading controls. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands from MB (n = 6) and ST (n = 3) are shown below the respective blots. They are represented as solid line for pJNK54 and JNK54, whereas pJNK46 and JNK46 are depicted as dotted lines in A and B. Activation of p38 MAPK and JNK is indicated by their respective pMAPK/MAPK ratio. The increase in ratio is expressed as fold increase with respect to the control ratio (1.0). Values are mean ± SD. Asterisks indicate values significantly different from corresponding control (p < 0.05). Repeated measures of ANOVA followed by Dunnet's test was performed for A and B, whereas paired t test was performed for C and D.
Figure 2.
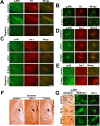
A–D, Activation of p38 in substantia nigra pars compacta (SNpc) and their terminals in the striatum after administration of MPTP to mice. Animals were treated with a single dose of MPTP and killed 24 h later or treated with a daily dose of MPTP for 8 d and killed on the ninth day. A, Immunohistochemical colocalization revealed the presence of pp38 (green) in the soma of tyrosine hydroxylase (TH) positive (red) SNpc neurons of the ventral midbrain after MPTP exposure. pp38 is present at low levels in the SNpc neurons of control animals while not detectable in ventral tegmental area (VTA) neurons. After single dose for 24 h and 8 d of MPTP treatment, there was an increase in pp38 in the SNpc but not VTA (arrowhead). Negative control for colocalization of pp38 and TH in the ventral midbrain is depicted. Corresponding magnified images of the neurons in SNpc (B) and VTA (C) are depicted. D, Images from the striatum show pp38 in the nerve terminals projecting toward the striatum in control, after a single dose and sub chronic exposure to MPTP. Scale bars: 25 μm; magnified images, 10 μm.
Figure 3.
Activation of JNK in microglial cells of the ventral midbrain and striatum after administration of MPTP to mice. Animals were treated with a single dose of MPTP and killed 24 h later or a daily dose of MPTP for 8 d and killed on the ninth day. A, Immunohistochemical colocalization revealed the presence of pJNK (green) throughout the brain including tyrosine hydroxylase positive neurons of the SNpc (red). Negative control for colocalization of pJNK and TH in the ventral midbrain is depicted. B, JNK activation was observed predominantly in the microglia in SNpc after 8 d of MPTP treatment. C, pJNK (green) colocalized with Iba1(red) after single and 8 d of MPTP treatment. Negative control for colocalization of pJNK and Iba1in the ventral midbrain is depicted. D, Magnified images depicting the colocalization of Iba1(red) with pJNK (green) after single dose and 8 d of MPTP treatment. E, pp38 does not colocalize with Iba1 after MPTP treatment. F, JNK activation was also observed specifically in microglia in the striatum after single dose and 8 d of MPTP treatment. The magnified representative images of cells indicated by arrowheads in F is depicted in G (first column). G, Change in morphology of cells positive for pJNK to microglial morphology in the ventral midbrain after MPTP treatment is also depicted. Neuron specific staining of pJNK is seen in the control section. Scale bars: 25 μm; magnified images, 10 μm.
Figure 4.
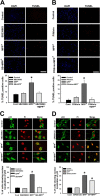
MPTP administration up-regulates p53 and induces p53 mediated transactivation, in vivo. Animals were treated with a single dose of vehicle or MPTP and killed 12 and 24 h later or with a daily dose of MPTP for 8 d and killed on the ninth day. A, pp53 (Ser15) accumulates in the nucleus 12 h after a single dose of MPTP and after subchronic exposure to MPTP for 8 d. Representative immunoblots of nuclear p53 and phospho-p53 Ser15 with their densitometric analyses are depicted. Values are mean ± SD (n = 7 animals). B, Representative immunoblot of extranuclear and cytosolic p53 in ventral midbrain (MB) and densitometric analyses of the post nuclear supernatant (PNS) and cytosolic p53 show the upregulation after MPTP exposure. Values are mean ± SD (n = 6 animals). C, Quantitative assessment of the expression of Noxa and Puma in ventral midbrain after MPTP treatment. qRT-PCR analysis using mouse ventral midbrain RNA shows relatively higher expression of Noxa and Puma in mouse ventral midbrain after MPTP treatment for 12 h. All samples were normalized using 18S rRNA expression. Values are mean ± SD (n = 6 animals). D, Representative immunoblot for Bax from ventral midbrain (MB; n = 5) of animals and densitometric analyses are shown. E, Animals were treated with a single dose of vehicle (3% DMSO) or MPTP and killed 12 h later. Some animals also received a single dose of SB239063 intrathecally. E–G, Representative blots from ventral midbrain of animals treated with DMSO (lane 1), MPTP (lane 2), SB239063 (lane 3), and SB239063 + MPTP (lane 4) depicting the protein levels of phospho-p38, p38, and Bax (G) in the extranuclear compartment and pp53 Ser15 and p53 (F) in the nuclear compartment as determined by immunoblot are presented. pp38/p38 ratio represents p38 activation in ventral midbrain. Densitometric analysis of the immunoblots representing the relative intensity of the immunoreactive bands are shown. Values are mean ± SD (n = 4 animals). β-Tubulin levels were measured as loading control for extranuclear compartment and lamin/histone levels were measured as loading control for nuclear compartment. Asterisks indicate values significantly different from corresponding control (p < 0.05). Statistical analysis using t test for A–E and repeated measures of ANOVA followed by Dunnet's test was performed for F–I.
Figure 5.
p38 inhibitor confers protection against MPP+ mediated cell death by preventing p53 translocation into the nucleus. A, Cells treated with SB239063 (1 μ
m
) for 60 min before exposure to MPP+ (10 μ
m
) for 24 h were protected from MPP+ mediated cell death. Scale bar, 200 μm. Cells were treated with either vehicle or SB239063 before the treatment with MPP+, quantification represents percentage of cells positive for TUNEL staining per total number of cells as represented by DAPI staining. B, p53 inhibitor pifithrin-α confers protection against MPP+ mediated cell death. Cells treated with pifithrin-α (250 n
m
) for 60 min before exposure to MPP+ (10 μ
m
) for 24 h were not affected by MPP+ mediated cell death. Scale bar, 200 μm. Cells were treated with either vehicle or pifithrin-α before the treatment with MPP+, quantification represents, percentage of cells positive for TUNEL staining per total number of cells as represented by DAPI count. C, p53, localized in the cytoplasm in primary neurons under control condition translocated into the nucleus in response to MPP+ exposure. The nuclear translocation was prevented by pretreating the cells with p38 inhibitor, SB239063. D, Nuclear translocation was partially prevented by pretreating the cells with JNK inhibitor, SP600125 (panel 4). Total number of cells is represented by propidium iodide (PI) staining. Data are represented as mean ± SD of four independent experiments. Asterisks indicate values significantly different from controls (p < 0.05).
Figure 6.
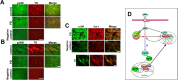
pp38 and pJNK in the substantia nigra of PD patients and schematic representation of the role of p38 in MPTP mediated toxicity. A, Immunohistochemical colocalization of phospho-p38 (green) in Tyrosine hydroxylase (red) positive neurons of control (traffic accident victims) and PD substantia nigra. B, Immunohistochemical colocalization of phospho-JNK (green) in Tyrosine hydroxylase (red) positive neurons of control (traffic accident victims) and PD substantia nigra. C, pJNK immunopositive cells colocalizes with Iba-1 positive cells of substantia nigra (green) in PD brain. The bottom depicts a magnified microglial cell indicating the colocalization of pJNK with Iba-1. Negative control was performed for each set of experiment. Scale bars: (B, C) 10 μm; (A) 25 μm. D, MPP+ is taken up by VMAT and concentrated in the synaptic vesicle generating ROS. MPP+ the toxic metabolite of MPTP causes increased production of ROS and mitochondrial dysfunction in dopaminergic neurons by inhibiting complex I of the electron transport chain. Mitochondrial complex I inhibition further contributes to ROS generation which presumably activates p38 MAP kinase pathway. As a consequence, the downstream target p53 is phosphorylated. p53 subsequently translocates to the nucleus transcriptionally activating the expression of Puma, Noxa and Bax. Bax translocates to the mitochondria ultimately leading to cell death. Approaches aimed at inhibiting p38 activation can help terminate this cascade and attenuate MPTP-induced neurodegeneration. DAT, Dopamine transporter; N, nucleus; VMAT, vesicular monoamine transporter.
Similar articles
- Activation of p38 MAPK in the substantia nigra leads to nuclear translocation of NF-kappaB in MPTP-treated mice: implication in Parkinson's disease.
Karunakaran S, Ravindranath V. Karunakaran S, et al. J Neurochem. 2009 Jun;109(6):1791-9. doi: 10.1111/j.1471-4159.2009.06112.x. Epub 2009 May 11. J Neurochem. 2009. PMID: 19457134 - Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: protection by alpha-lipoic acid.
Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Karunakaran S, et al. FASEB J. 2007 Jul;21(9):2226-36. doi: 10.1096/fj.06-7580com. Epub 2007 Mar 16. FASEB J. 2007. PMID: 17369508 - p38(MAPK)/p53-Mediated Bax induction contributes to neurons degeneration in rotenone-induced cellular and rat models of Parkinson's disease.
Wu F, Wang Z, Gu JH, Ge JB, Liang ZQ, Qin ZH. Wu F, et al. Neurochem Int. 2013 Sep;63(3):133-40. doi: 10.1016/j.neuint.2013.05.006. Epub 2013 May 25. Neurochem Int. 2013. PMID: 23714208 - A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson's disease and potential molecular targets.
Ramsey CP, Tansey MG. Ramsey CP, et al. Exp Neurol. 2014 Jun;256:126-32. doi: 10.1016/j.expneurol.2013.05.014. Epub 2013 May 28. Exp Neurol. 2014. PMID: 23726958 Free PMC article. Review. - Glutathione metabolism and Parkinson's disease.
Smeyne M, Smeyne RJ. Smeyne M, et al. Free Radic Biol Med. 2013 Sep;62:13-25. doi: 10.1016/j.freeradbiomed.2013.05.001. Epub 2013 May 8. Free Radic Biol Med. 2013. PMID: 23665395 Free PMC article. Review.
Cited by
- Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: a role of NADPH oxidase 2.
Cahill-Smith S, Li JM. Cahill-Smith S, et al. Br J Clin Pharmacol. 2014 Sep;78(3):441-53. doi: 10.1111/bcp.12357. Br J Clin Pharmacol. 2014. PMID: 25279404 Free PMC article. Review. - Cell cycle inhibition without disruption of neurogenesis is a strategy for treatment of aberrant cell cycle diseases: an update.
Liu DZ, Ander BP. Liu DZ, et al. ScientificWorldJournal. 2012;2012:491737. doi: 10.1100/2012/491737. Epub 2012 Apr 1. ScientificWorldJournal. 2012. PMID: 22547985 Free PMC article. Review. - p53 Plays an important role in cell fate determination after exposure to microcystin-LR.
Takumi S, Komatsu M, Furukawa T, Ikeda R, Sumizawa T, Akenaga H, Maeda Y, Aoyama K, Arizono K, Ando S, Takeuchi T. Takumi S, et al. Environ Health Perspect. 2010 Sep;118(9):1292-8. doi: 10.1289/ehp.1001899. Epub 2010 Apr 26. Environ Health Perspect. 2010. PMID: 20421190 Free PMC article. - NADPH ameliorates MPTP-induced dopaminergic neurodegeneration through inhibiting p38MAPK activation.
Zhou JS, Zhu Z, Wu F, Zhou Y, Sheng R, Wu JC, Qin ZH. Zhou JS, et al. Acta Pharmacol Sin. 2019 Feb;40(2):180-191. doi: 10.1038/s41401-018-0003-0. Epub 2018 May 16. Acta Pharmacol Sin. 2019. PMID: 29769744 Free PMC article. - α-Synucleinopathy associated c-Abl activation causes p53-dependent autophagy impairment.
Karim MR, Liao EE, Kim J, Meints J, Martinez HM, Pletnikova O, Troncoso JC, Lee MK. Karim MR, et al. Mol Neurodegener. 2020 Apr 16;15(1):27. doi: 10.1186/s13024-020-00364-w. Mol Neurodegener. 2020. PMID: 32299471 Free PMC article.
References
- Barden H. The oxidative generation of sulfonic acid groups in neuromelanin and lipofuscin in the human brain. J Histochem Cytochem. 1984;32:329–336. - PubMed
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Cao FL, Liu MG, Hao J, Li Z, Lu ZM, Chen J. Different roles of spinal p38 and c-Jun N-terminal kinase pathways in bee venom-induced multiple pain-related behaviors. Neurosci Lett. 2007;427:50–54. - PubMed
- Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. - PubMed
- Ferrer I, Blanco R, Carmona M, Puig B, Barrachina M, Gómez C, Ambrosio S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and Dementia with Lewy bodies. J Neural Transm. 2001;108:1383–1396. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous