Modification of HER2 pre-mRNA alternative splicing and its effects on breast cancer cells - PubMed (original) (raw)
Modification of HER2 pre-mRNA alternative splicing and its effects on breast cancer cells
Jing Wan et al. Int J Cancer. 2009.
Abstract
The oncogene HER2 is overexpressed in a variety of human tumors, providing a target for anti-cancer molecular therapies. Here, we employed a 2'-O-methoxyethyl (MOE) splice switching oligonucleotide, SSO111, to induce skipping of exon 15 in HER2 pre-mRNA, leading to significant downregulation of full-length HER2 mRNA, and simultaneous upregulation of Delta15HER2 mRNA. SSO111 treatment of SK-BR-3 cells, which highly overexpress HER2, led to inhibition of cell proliferation and induction of apoptosis. The novel Delta15HER2 mRNA encodes a soluble, secreted form of the receptor. Treating SK-BR-3 cells with exogenous Delta15HER2 protein reduced membrane-bound HER2 and decreased HER3 transphosphorylation. Delta15HER2 protein thus has similar activity to an autoinhibitory, natural splice variant of HER2, Herstatin, and to the breast cancer drug Herceptin. Both SSO111 and Delta15HER2 may be potential candidates for the development of novel HER2-targeted cancer therapeutics.
Conflict of interest statement
Financial disclosure: RK declares a potential conflict of interest as an employee of AVI BioPharma, Inc.
Figures
Figure 1
Skipping of exon 15 in HER2 pre-mRNA. A) SSOs were designed to target the 5’ splice site of exon 15. Skipping of exon 15 induced by SSOs generated a downstream stop codon, and results in a truncated HER2 ECD. Thick bar: SSO. Part of HER2 pre-mRNA (3’ to 5’, with intron underlined), SSO111, and SSO111m (with mismatches underlined and highlighted in bold) were shown in B). B) and C) Results from the analysis of total cellular RNA by RT-PCR are shown: B) Dose-dependence of SK-BR-3 cells treated with SSO111 at designated concentrations for 24 hours; C) Time-course of Sk-BR-3 cells treated with SSO111 for the designated time at 100 nM. The lengths of the PCR products (in base pairs) are indicated. Lower panels in B) and C): quantitative analysis of the results. Gray bars: SSO111 transfected cells; white bars: 111m-transfected cells.
Figure 2
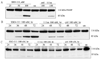
Western blot analysis of HER2 protein from SK-BR-3 cells transfected with SSO111. A) dose dependence (at 48 hours). B) time course (at 100 nM). Top panels: HER2 protein; lower panels: β-actin as a loading control. See “Methods and Materials” for details.
Figure 3
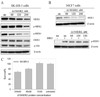
Growth inhibition of SK-BR-3 cells by SSO111 treatment. A) Western blot demonstrating relative HER2 expression in MCF7 and SK-BR-3 cell lines (20 µg protein/lane). B) MTS assay of SK-BR-3 (black bar) and MCF7 (gray bar) cells after 72 hours incubation with 100 nM SSO111. Shown are the mean ± standard deviation of triplicates.
Figure 4
PARP cleavage as a marker for apoptosis after SSO111 treatment. In SK-BR-3 cells: A) dose dependence (at 48 hours); B) time course (at 100 nM). In MCF7 cells: C) time course (at 100 nM). Full-length PARP: 116 kDa; cleavage product: 85 kDa.
Figure 5
Effects of Δ15HER2-His protein on HER2/HER3 receptors. Western blot analysis of (A) SK-BR-3 and (B) MCF7 cell lysates after treatment with purified Δ15HER2-His protein at the designated concentrations. For analysis of phosphorylated Akt (p-Akt) in A) and phosphorylated HER3 (p-HER3) protein in B), cells were stimulated with heregulin (15 min, 20 ng/ml). C) Growth inhibition of SK-BR-3 cells by Δ15HER2-His protein treatment after 72 hours incubation analyzed by MTS assay. Shown are the mean ± standard deviation of triplicates.
Figure 6
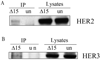
Interaction between Δ15HER2-His protein and HER2/HER3. A) SK-BR-3 and B) MCF7 cell lysates were collected 48 hours after transfection with Δ15HER2-His plasmid, the protein was immunoprecipitated with anti-His tag antibody and blotted with anti-HER2 antibody and anti-HER3 antibody, respectively.
Similar articles
- How is Herstatin, a tumor suppressor splice variant of the oncogene HER2, regulated?
Silipo M, Gautrey H, Satam S, Lennard T, Tyson-Capper A. Silipo M, et al. RNA Biol. 2017 May 4;14(5):536-543. doi: 10.1080/15476286.2016.1267074. Epub 2016 Dec 9. RNA Biol. 2017. PMID: 27935425 Free PMC article. - Antisense-mediated exon skipping to shift alternative splicing to treat cancer.
Wan J. Wan J. Methods Mol Biol. 2012;867:201-8. doi: 10.1007/978-1-61779-767-5_13. Methods Mol Biol. 2012. PMID: 22454063 - Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer.
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Nahta R, et al. Nat Clin Pract Oncol. 2006 May;3(5):269-80. doi: 10.1038/ncponc0509. Nat Clin Pract Oncol. 2006. PMID: 16683005 Review. - Mechanism of action of anti-HER2 monoclonal antibodies.
Baselga J, Albanell J. Baselga J, et al. Ann Oncol. 2001;12 Suppl 1:S35-41. doi: 10.1093/annonc/12.suppl_1.s35. Ann Oncol. 2001. PMID: 11521720 Review.
Cited by
- Aptamer-mediated delivery of splice-switching oligonucleotides to the nuclei of cancer cells.
Kotula JW, Pratico ED, Ming X, Nakagawa O, Juliano RL, Sullenger BA. Kotula JW, et al. Nucleic Acid Ther. 2012 Jun;22(3):187-95. doi: 10.1089/nat.2012.0347. Nucleic Acid Ther. 2012. PMID: 22703281 Free PMC article. - Increasing the relative expression of endogenous non-coding Steroid Receptor RNA Activator (SRA) in human breast cancer cells using modified oligonucleotides.
Cooper C, Guo J, Yan Y, Chooniedass-Kothari S, Hube F, Hamedani MK, Murphy LC, Myal Y, Leygue E. Cooper C, et al. Nucleic Acids Res. 2009 Jul;37(13):4518-31. doi: 10.1093/nar/gkp441. Epub 2009 May 29. Nucleic Acids Res. 2009. PMID: 19483093 Free PMC article. - AS1411-functionalized delivery nanosystems for targeted cancer therapy.
Kozani PS, Kozani PS, Malik MT. Kozani PS, et al. Explor Med. 2021;2:146-166. doi: 10.37349/emed.2021.00039. Epub 2021 Apr 30. Explor Med. 2021. PMID: 34723284 Free PMC article. - Antisense Modulation of RNA Processing as a Therapeutic Approach in Cancer Therapy.
Spraggon L, Cartegni L. Spraggon L, et al. Drug Discov Today Ther Strateg. 2013 Fall;10(3):e139-e148. doi: 10.1016/j.ddstr.2013.06.002. Drug Discov Today Ther Strateg. 2013. PMID: 25589899 Free PMC article. - SplicerAV: a tool for mining microarray expression data for changes in RNA processing.
Robinson TJ, Dinan MA, Dewhirst M, Garcia-Blanco MA, Pearson JL. Robinson TJ, et al. BMC Bioinformatics. 2010 Feb 25;11:108. doi: 10.1186/1471-2105-11-108. BMC Bioinformatics. 2010. PMID: 20184770 Free PMC article.
References
- Hynes N, Stern D. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochem. Biophys. Acta. 1994;1198:165–184. - PubMed
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61:1–13. - PubMed
- Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim. Biophys. Acta. 1998;1377:M25–M37. - PubMed
- Lonardo F, Di Marco E, King C, Pierce J, Segatto O, Aaronson S, Di Fiore P. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. The New Biologist. 1990;2:992–1003. - PubMed
- Rubin I, Yarden Y. The basic biology of HER2. Annals of Oncology. 2001;12 Suppl. 1:S3–S8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous