Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro - PubMed (original) (raw)
Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro
Qixuan Chen et al. Nutrition. 2009 Mar.
Abstract
Objective: A growing body of evidence supports an antiobesity effect of dairy products; however, the mechanisms remain unclear. The objective of this study was to explore possible intestinal mechanisms by which dairy delivers an antiobesity effect. The human intestinal cell line, NCI-H716, was used to test the hypothesis that branched-chain amino acids and dairy proteins regulate satiety hormone secretion and modulate genes involved in fatty acid and cholesterol metabolism.
Methods: In dose-response (0.5%, 1.0%, 2.0%, and 3.0%) studies, the effect of leucine, isoleucine, valine, skim milk, casein, and whey on glucagon-like peptide-1 release and the expression of selected genes were tested.
Results: Leucine, isoleucine, skim milk, and casein stimulated glucagon-like peptide-1 release (P < 0.05). Isoleucine and whey downregulated the expression of intestinal-type fatty acid binding protein (i-FABP), fatty acid transport protein 4 (FATP4), Niemann-Pick C-1-like-1 protein (NPC1L1), acetyl-coenzyme A carboxylase (ACC), fatty acid synthase (FAS), sterol regulatory element-binding protein-2 (SREBP-2), and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR; P < 0.05). Leucine and valine downregulated the expression of NPC1L1, ACC, FAS, SREBP-2, and HMGCR (P < 0.05). Casein downregulated the expression of i-FABP, FATP4, ACC, FAS, SREBP-2, and HMGCR (P < 0.05). Skim milk downregulated the expression of ACC, FAS, and SREBP-2, but not i-FABP, FATP4, and NPC1L1.
Conclusion: This work suggests that the antiobesity effect of dairy may be mediated, at least in part, by integration of events that promote glucagon-like peptide-1 secretion and inhibit expression of genes involved in intestinal fatty acid and cholesterol absorption and synthesis.
Figures
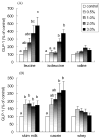
Fig. 1
Effects of branched-chain amino acids, skim milk, casein, and whey on GLP-1 release in NCI-H716 cells. Cells (1 × 106) were incubated for 2 h with increasing concentrations of (A) leucine, isoleucine, valine and (B) skim milk, casein, and whey. Secretion into the medium was normalized to the total cellular content and expressed as a percentage of the control value. Due to lower solubility, leucine concentrations were 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four independent experiments. Treatments with different letters are significantly different (P < 0.05). GLP-1, glucagon-like peptide-1.
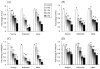
Fig. 2
Effects of leucine, isoleucine, and valine on in vitro mRNA expression of (A) i-FABP, (B) FATP4, and (C) NPC1L1. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Polymerase chain reaction products for genes of interest were normalized to _β_-actin mRNA as a control. Due to lower solubility, leucine concentrations are 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). FATP4, fatty acid transport protein-4; i-FAB, intestinal-type fatty acid binding protein; NPC1L1, Niemann-Pick C-1–like-1 protein.
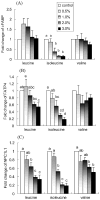
Fig. 3
Effect of leucine, isoleucine, and valine on in vitro mRNA expression of (A) ACC, (B) FAS, (C) SREBP-2, and (D) HMGCR. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Due to lower solubility, leucine concentrations are 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; SREBP-2, sterol regulatory element binding protein-2.
Fig. 4
Effects of skim milk, casein, and whey on in vitro mRNA expression of (A) i-FABP, (B) FATP4, and (C) NPC1L1. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). FATP4, fatty acid transport protein-4; i-FABP, intestinal-type fatty acid binding protein; NPC1L1, Niemann-Pick C-1–like-1 protein.
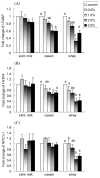
Fig. 5
Effects of skim milk, casein, and whey on in vitro mRNA expression of (A) ACC, (B) FAS, (C) SREBP-2, and (D) HMGCR. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; HMGCR, 3-hydroxy-3-methylglutaryl-cenzyme A reductase; SREBP-2, sterol regulatory element binding protein-2.
Similar articles
- Dairy proteins, dairy lipids, and postprandial lipemia in persons with abdominal obesity (DairyHealth): a 12-wk, randomized, parallel-controlled, double-blinded, diet intervention study.
Bohl M, Bjørnshave A, Rasmussen KV, Schioldan AG, Amer B, Larsen MK, Dalsgaard TK, Holst JJ, Herrmann A, O'Neill S, O'Driscoll L, Afman L, Jensen E, Christensen MM, Gregersen S, Hermansen K. Bohl M, et al. Am J Clin Nutr. 2015 Apr;101(4):870-8. doi: 10.3945/ajcn.114.097923. Epub 2015 Jan 14. Am J Clin Nutr. 2015. PMID: 25833983 Clinical Trial. - Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells.
Kim B, Park Y, Wegner CJ, Bolling BW, Lee J. Kim B, et al. J Nutr Biochem. 2013 Sep;24(9):1564-70. doi: 10.1016/j.jnutbio.2013.01.005. Epub 2013 Mar 18. J Nutr Biochem. 2013. PMID: 23517916 - Whey protein hydrolysate and branched-chain amino acids downregulate inflammation-related genes in vascular endothelial cells.
Da Silva MS, Bigo C, Barbier O, Rudkowska I. Da Silva MS, et al. Nutr Res. 2017 Feb;38:43-51. doi: 10.1016/j.nutres.2017.01.005. Epub 2017 Jan 23. Nutr Res. 2017. PMID: 28381353 - Comparative Effects of the Branched-Chain Amino Acids, Leucine, Isoleucine and Valine, on Gastric Emptying, Plasma Glucose, C-Peptide and Glucagon in Healthy Men.
Elovaris RA, Bitarafan V, Agah S, Ullrich SS, Lange K, Horowitz M, Feinle-Bisset C. Elovaris RA, et al. Nutrients. 2021 May 11;13(5):1613. doi: 10.3390/nu13051613. Nutrients. 2021. PMID: 34064996 Free PMC article. - Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns.
Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Chatterton DE, et al. Int J Biochem Cell Biol. 2013 Aug;45(8):1730-47. doi: 10.1016/j.biocel.2013.04.028. Epub 2013 May 6. Int J Biochem Cell Biol. 2013. PMID: 23660296 Review.
Cited by
- The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults.
Newmire DE, Rivas E, Deemer SE, Willoughby DS, Ben-Ezra V. Newmire DE, et al. Nutrients. 2019 Nov 4;11(11):2650. doi: 10.3390/nu11112650. Nutrients. 2019. PMID: 31689951 Free PMC article. - Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization.
Melnik BC, John SM, Schmitz G. Melnik BC, et al. J Transl Med. 2015 Jan 16;13:13. doi: 10.1186/s12967-014-0377-9. J Transl Med. 2015. PMID: 25592553 Free PMC article. Review. - Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice.
Shin CY, Lee HY, Kim GH, Park SY, Choi WS, Sohn UD. Shin CY, et al. Biomol Ther (Seoul). 2021 Jul 1;29(4):419-426. doi: 10.4062/biomolther.2021.003. Biomol Ther (Seoul). 2021. PMID: 33814417 Free PMC article. - Dairy products and plasma cholesterol levels.
Ohlsson L. Ohlsson L. Food Nutr Res. 2010 Aug 19;54. doi: 10.3402/fnr.v54i0.5124. Food Nutr Res. 2010. PMID: 20806084 Free PMC article. - Intraduodenal milk protein concentrate augments the glycemic and food intake suppressive effects of DPP-IV inhibition.
Olivos DR, McGrath LE, Turner CA, Montaubin O, Mietlicki-Baase EG, Hayes MR. Olivos DR, et al. Am J Physiol Regul Integr Comp Physiol. 2014 Feb 1;306(3):R157-63. doi: 10.1152/ajpregu.00358.2013. Epub 2013 Dec 18. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24352410 Free PMC article.
References
- Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82:523–30. - PubMed
- Dixon LB, Pellizzon MA, Jawad AF, Tershakovec AM. Calcium and dairy intake and measures of obesity in hyper- and normocholesterolemic children. Obes Res. 2005;13:1727–38. - PubMed
- Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83:559–66. - PubMed
- Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G, et al. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn Study Am J Clin Nutr. 2007;85:989–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous