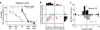Representation of negative motivational value in the primate lateral habenula - PubMed (original) (raw)
Representation of negative motivational value in the primate lateral habenula
Masayuki Matsumoto et al. Nat Neurosci. 2009 Jan.
Abstract
An action may lead to either a reward or a punishment. Therefore, an appropriate action needs to be chosen on the basis of the values of both expected rewards and expected punishments. To understand the underlying neural mechanisms, we conditioned monkeys using a Pavlovian procedure with two distinct contexts: one in which rewards were available and another in which punishments were feared. We found that the population of lateral habenula neurons was most strongly excited by a conditioned stimulus associated with the most unpleasant event in each context: the absence of the reward or the presence of the punishment. The population of lateral habenula neurons was also excited by the punishment itself and inhibited by the reward itself, especially when they were less predictable. These results suggest that the lateral habenula has the potential to adaptively control both reward-seeking and punishment-avoidance behaviors, presumably through its projections to dopaminergic and serotonergic systems.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures
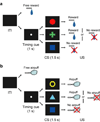
Figure 1
Pavlovian procedure with two distinct contexts. (a) Reward block. (b) Punishment block.
Figure 2
Responses of lateral habenula neurons to CSs. (a) Activity of an example neuron during the reward block. Rasters and spike density functions (SDFs) are aligned by CS onset and shown for 100% reward CS, 50% reward CS, and 0% reward CS. (b) Activity of the same neuron in a during the punishment block. Rasters and SDFs are shown for 100% airpuff CS, 50% airpuff CS, and 0% airpuff CS. (c) Averaged activity of the 49 neurons during the reward block. SDFs are shown for 100% reward CS (dark red), 50% reward CS (light red), and 0% reward CS (gray). Gray area indicates the period that was used to analyze CS response. (d) Averaged activity of the 49 neurons during the punishment block. SDFs are shown for 100% airpuff CS (dark blue), 50% airpuff CS (light blue), and 0% airpuff CS (gray).
Figure 3
Relation between objective value and CS response. (a) Averaged magnitude of the CS response of the 49 neurons plotted against the objective value of outcome for the reward block (black) and the punishment block (gray). Filled symbols indicate a significant deviation from zero (P < 0.05, Wilcoxon signed-rank test). Double asterisks indicate a significant difference between two CS responses (P < 0.01, Wilcoxon signed-rank test). Error bars indicate s.e.m. (b) Correlation coefficients of the 49 neurons between objective value and CS response. The abscissa indicates correlation coefficient between reward value and CS response. The ordinate indicates correlation coefficient between airpuff value and CS response. Cyan, dark blue and magenta plots indicate neurons with statistically significant correlation between reward value and CS response, between airpuff value and CS response, and both of them, respectively (P < 0.05). White plots, no significance. The marginal histograms show the distribution of correlation coefficients. Black bars indicate neurons with statistically significant correlation (P < 0.05). White bars, no significance. (c) Distributions of the linearity indices of the 49 neurons. Black bars indicate the distribution of linearity indices in the reward block. Gray bars indicate the distribution in the punishment block.
Figure 4
Changes in the averaged responses of the 49 neurons to 0% reward CS (black) and 0% airpuff CS (gray) after the block context was reversed. The abscissa indicates the number of preceding trials (excluding 0% reward CS and 0% airpuff CS trials) in a given block. When either 0% reward CS and 0% airpuff CS was presented on the first trial after block change, the neuronal response was included in the data at trial zero. Error bars indicate s.e.m.
Figure 5
Responses of lateral habenula neurons to USs. (a) Activity of an example neuron during the reward block. Rasters and SDFs are aligned by reward onset and shown for 100% reward, 50% reward, and free reward. (b) Activity of the same neuron in a during the punishment block. Rasters and SDFs are aligned by airpuff onset and shown for 100% airpuff, 50% airpuff, and free airpuff. (c) Averaged activity of the 51 neurons showing a significant response to at least one of 100% reward (dark red), 50% reward (light red), and free reward (gray) (P < 0.05, Wilcoxon signed-rank test). Gray area indicates the period that was used to analyze US response. (d) Averaged activity of the 60 neurons showing a significant response to at least one of 100% airpuff (dark blue), 50% airpuff (light blue), and free airpuff (gray) (P < 0.05, Wilcoxon signed-rank test).
Figure 6
Responses of lateral habenula neurons to US omission. (a) Activity of the same neuron in Figures 5a and b during the reward block. Rasters and SDFs are aligned by CS offset which occurred simultaneously with reward onset in rewarded trials, and shown for 50% reward omission and 0% reward omission. (b) Activity of the same neuron in a during the punishment block. Rasters and SDFs are aligned by CS offset which occurred simultaneously with airpuff onset in airpuff trials, and shown for 50% airpuff omission and 0% airpuff omission. (c) Averaged activity of the 51 neurons for 50% reward omission (light red) and 0% reward omission (gray). Gray area indicates the period that was used to analyze US omission response. (d) Averaged activity of the 60 neurons for 50% airpuff omission (light blue) and 0% airpuff omission (gray).
Figure 7
Relation between prediction error and US and US omission responses. (a) Averaged magnitude of the US and US omission responses of the 51 neurons plotted against prediction error for reward. Filled symbols indicate a significant deviation from zero (P < 0.05, Wilcoxon signed-rank test). Double asterisks indicate a significant difference between two responses (P < 0.01, Wilcoxon signed-rank test). Error bars indicate s.e.m. (b) Averaged magnitude of the US and US omission responses of the 60 neurons plotted against prediction error for airpuff. Conventions are the same as a. (c) Correlation coefficients of all 72 neurons between prediction error and US and US omission responses. The abscissa indicates the correlation coefficient between reward prediction error and US and US omission responses. The ordinate indicates the correlation coefficient between airpuff prediction error and US and US omission responses. Cyan, dark blue and magenta plots indicate neurons with statistically significant correlation between reward prediction error and US and US omission responses, between airpuff prediction error and US and US omission responses, and both of them, respectively (P < 0.05). White plots, no significance. The marginal histograms show the distribution of correlation coefficients. Black bars indicate neurons with statistically significant correlation (P < 0.05). White bars, no significance.
Figure 8
Comparison between CS response and US response. (a) Comparison between the response to 100% reward CS and the response to free reward for all 72 neurons. Dark blue, cyan and magenta dots indicate neurons with statistically significant responses to free reward, 100% reward CS, and both of them, respectively (P < 0.05, Wilcoxon signed-rank test). (b) Comparison between the response to 100% airpuff CS and the response to free airpuff for all 72 neurons. Dark blue, cyan and magenta dots indicate neurons with statistically significant responses to free airpuff, 100% airpuff CS, and both of them, respectively (P < 0.05, Wilcoxon signed-rank test).
Similar articles
- Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons.
Bromberg-Martin ES, Matsumoto M, Hikosaka O. Bromberg-Martin ES, et al. Neuron. 2010 Jul 15;67(1):144-55. doi: 10.1016/j.neuron.2010.06.016. Neuron. 2010. PMID: 20624598 Free PMC article. - Learning shapes the aversion and reward responses of lateral habenula neurons.
Wang D, Li Y, Feng Q, Guo Q, Zhou J, Luo M. Wang D, et al. Elife. 2017 May 31;6:e23045. doi: 10.7554/eLife.23045. Elife. 2017. PMID: 28561735 Free PMC article. - Anterior cingulate is a source of valence-specific information about value and uncertainty.
Monosov IE. Monosov IE. Nat Commun. 2017 Jul 26;8(1):134. doi: 10.1038/s41467-017-00072-y. Nat Commun. 2017. PMID: 28747623 Free PMC article. - [Role of the lateral habenula and dopamine neurons in reward processing].
Matsumoto M. Matsumoto M. Brain Nerve. 2009 Apr;61(4):389-96. Brain Nerve. 2009. PMID: 19378808 Review. Japanese. - Reward processing by the lateral habenula in normal and depressive behaviors.
Proulx CD, Hikosaka O, Malinow R. Proulx CD, et al. Nat Neurosci. 2014 Sep;17(9):1146-52. doi: 10.1038/nn.3779. Nat Neurosci. 2014. PMID: 25157511 Free PMC article. Review.
Cited by
- Defining the habenula in human neuroimaging studies.
Lawson RP, Drevets WC, Roiser JP. Lawson RP, et al. Neuroimage. 2013 Jan 1;64:722-7. doi: 10.1016/j.neuroimage.2012.08.076. Epub 2012 Sep 8. Neuroimage. 2013. PMID: 22986224 Free PMC article. - Molecular regionalization of the diencephalon.
Martinez-Ferre A, Martinez S. Martinez-Ferre A, et al. Front Neurosci. 2012 May 25;6:73. doi: 10.3389/fnins.2012.00073. eCollection 2012. Front Neurosci. 2012. PMID: 22654731 Free PMC article. - Neuronal reference frames for social decisions in primate frontal cortex.
Chang SW, Gariépy JF, Platt ML. Chang SW, et al. Nat Neurosci. 2013 Feb;16(2):243-50. doi: 10.1038/nn.3287. Epub 2012 Dec 23. Nat Neurosci. 2013. PMID: 23263442 Free PMC article. - Predictions about reward outcomes in rhesus monkeys.
Huang Y, Chang H, Santos LR, Rosati AG. Huang Y, et al. Behav Neurosci. 2024 Feb;138(1):43-58. doi: 10.1037/bne0000573. Epub 2023 Dec 7. Behav Neurosci. 2024. PMID: 38060026 Free PMC article. - Multiple timescales of memory in lateral habenula and dopamine neurons.
Bromberg-Martin ES, Matsumoto M, Nakahara H, Hikosaka O. Bromberg-Martin ES, et al. Neuron. 2010 Aug 12;67(3):499-510. doi: 10.1016/j.neuron.2010.06.031. Neuron. 2010. PMID: 20696385 Free PMC article.
References
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. - PubMed
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. - PubMed
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. - PubMed
- Nieuwenhuis S, et al. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25:1302–1309. - PubMed
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources