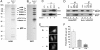Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase - PubMed (original) (raw)
Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase
Qiming Sun et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Autophagy mediates the cellular response to nutrient deprivation, protein aggregation, and pathogen invasion in human. Dysfunction of autophagy has been implicated in multiple human diseases including cancer. The identification of novel autophagy factors in mammalian cells will provide critical mechanistic insights into how this complicated cellular pathway responds to a broad range of challenges. Here, we report the cloning of an autophagy-specific protein that we called Barkor (Beclin 1-associated autophagy-related key regulator) through direct interaction with Beclin 1 in the human phosphatidylinositol 3-kinase class III complex. Barkor shares 18% sequence identity and 32% sequence similarity with yeast Atg14. Elimination of Barkor expression by RNA interference compromises starvation- and rapamycin-induced LC3 lipidation and autophagosome formation. Overexpression of Barkor leads to autophagy activation and increased number and enlarged volume of autophagosomes. Tellingly, Barkor is also required for suppression of the autophagy-mediated intracellular survival of Salmonella typhimurium in mammalian cells. Mechanistically, Barkor competes with UV radiation resistance associated gene product (UVRAG) for interaction with Beclin 1, and the complex formation of Barkor and Beclin1 is required for their localizations to autophagosomes. Therefore, we define a regulatory signaling pathway mediated by Barkor that positively controls autophagy through Beclin 1 and represents a potential target for drug development in the treatment of human diseases implicated in autophagic dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Barkor is a major component of the Beclin 1–PI3KC3 complex. (A) Silver staining of the tandem affinity-purified Beclin 1 complex or vector alone in U2OS cells. All the marked bands were identified by mass spectrometry. (B) A similar Beclin 1 complex was purified from human kidney embryonic HEK293T cells. (C) Reciprocal coimmunoprecipitation of Barkor and Beclin 1. 293T cell extracts were immunoprecipitated with either anti-Barkor or Beclin 1 antibody and then analyzed. (D) Beclin 1 bridges the interaction between PI3KC3 and Barkor. Beclin 1-knockdown 293T cells or control cells were transfected with FLAG-PI3KC3 and Myc-Barkor. Whole-cell lysates were immunoprecipitated with anti-FLAG or Myc antibodies and analyzed. (E) Barkor-knockdown decreases the activity of PI3KC3 in vivo. Barkor-knockdown U2OS cells were transfected with FYVE2-EGFP expression vector. Thirty hours after transfection, cells were treated with 5 mM 3-MA for another 4 h. FYVE2-EGFP was quantified in F.
Fig. 2.
Barkor is required for LC3 lipidation and autophagosome formation. (A) LC3 conjugation was examined in Beclin 1 and Barkor-knockdown U2OS cells in complete medium (DMEM + 10% FBS) or starvation medium (Earle's balanced salt solution, EBSS). (B) LC3 conjugation was examined in Barkor-knockdown cells treated with 500 nM rapamycin overnight. Proteases inhibitors (2 μg/mL E64D and 2 μg/mL pepstatin for 4 h) were used to block lysosomal degradation. (C–E). Electron microscopic (EM) analysis of Barkor-knockdown cells. Both control cells (C) and Barkor-knockdown cells (D) were starved in EBSS for 1 h and analyzed by transmission electron microscopy. (E) High-magnification picture of the framed area in C showing AVs (marked by arrows) that contain intracellular contents. [Scale bars: 2 μM (C), 2 μM (D), and 1 μM (E).] (F) AVs per cross-sectioned cell (mean ± SD; n = 21) under EM were calculated and summarized. CM, complete medium. Arrows indicate autophagic vacuole. (G–I) Barkor-overexpression (OE) U2OS cells (H and I) and U2OS parental cells (G) were observed under EM. (I) High-magnification picture of the framed area in H shows AVs (marked by arrows) that contain intracellular contents. [Scale bars: 1 μM (G–I).] (J) AVs per cross-sectioned cell under EM were calculated. (K) The average size of AVs in Barkor OE cells or normal cells was calculated and summarized. (L) HEK293T cells were transfected with Barkor (wild-type or CCD deletion mutant) or UVRAG, and LC3 conjugation was examined in these cells.
Fig. 3.
Barkor is indispensable for autophagy-mediated suppression of bacterial replication in vivo. (A) Atg7+/+ _and Atg7_−/− MEFs cells were infected with wild-type GFP-marked S. typhimurium (SL1344) (green) for 8 h and analyzed by immunostaining. Cells were counterstained with anti-tubulin antibody (red). (B) Atg7+/+ or _Atg7_−/− MEFs were infected with S. typhimurium (SL1344) for indicated times. The infected cells were treated with gentamicin sulfate to block extracellular bacterial amplification and then lysed, and internalized bacteria were plated on Petri dishes. The replication of bacteria was quantified by counting the colony number on the Petri plates. (C) Barkor-knockdown U2OS cells were induced by doxycycline for 2 days and infected with S. typhimurium as described in A. (D) The bacterial growth in Barkor-knockdown U2OS cells was measured as described in B.
Fig. 4.
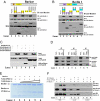
Barkor and UVRAG form distinct subcomplexes with Beclin 1 through their CCDs. (A) Barkor binds to Beclin 1 through its CCD. 293T cells were transfected with FLAG-Beclin 1, Myc-Barkor, or its Myc-tagged mutants. Whole-cell lysates (WCLs) were immunoprecipitated (IP) with anti-Myc followed by immunoblotting (IB) with anti-FLAG. § indicates a nonspecific band. (B) Beclin 1 binds to Barkor through its CCD. 293T cells were transfected with Myc-Barkor, FLAG-Beclin 1, or its FLAG-tagged mutants. WCLs were immunoprecipitated with anti-FLAG followed by the IB with anti-Myc. (C) Direct interaction of Beclin 1 with Barkor or UVRAG. A Ni-column was incubated first with His-Beclin 1-CC and then with FLAG-tagged Beclin 1-CC, Barkor-CC, and UVRAG-CC. Proteins bound to beads and inputs were analyzed. (D) Barkor and UVRAG form distinct subcomplexes with Beclin 1. 293T cells were transfected with FLAG-UVRAG, Myc-Barkor, and HA-Beclin 1. WCLs were immunoprecipitated with anti-FLAG, anti-HA, or Myc, and the immunoprecipitates were analyzed. (E) Barkor competes with UVRAG for binding to Beclin 1. UVRAG was first incubated with Beclin 1 in vitro; increasing doses of the Barkor CCD were then added to the reactions. After extensive washing, the proteins bound to beads were analyzed by SDS/PAGE stained with Coomassie blue. (F) UVRAG competes with Barkor–Beclin 1 interaction in vivo. HA-Beclin 1 was cotransfected into HEK293T cells with Myc-Barkor or FLAG-tagged UVRAG. WCLs were immunoprecipitated with anti-HA followed by the IB with antibodies against Myc, FLAG, or HA.
Fig. 5.
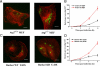
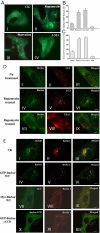
Barkor promotes Beclin 1 translocation to autophagosomes through direct interaction. (A) Subcellular localization of Barkor. Fluorescent Barkor-EGFP detected in transfected U2OS cells upon mock treatment (I), 500 nM rapamycin (II), EBSS medium (III), or EBSS and 5 mM 3-MA, respectively, under a fluorescence microscopy. (B) Quantification of Barkor-EGFP dots per cell. (C) Quantification of Barkor-EGFP punctate staining-positive cells. (D) Colocalization of Barkor and LC3. A U2OS stable cell line expressing Myc-LC3 was transfected with Barkor-EGFP and then mock treated (I–III) or treated with 500 nM rapamycin (IV–IX) for 12 h. (I–VI) GFP-Barkor (green) was costained with Myc-LC3 (red). (VII–IX) GFP-Barkor (green) was costained with endogenous EEA1 (red) (an endosome marker). (E) U2OS cells were transfected with RFP-Beclin 1. (I–III) RFP-Beclin 1 was costained with endogenous TGN38 (green) (a _trans_-Golgi network marker). (IV–VI) U2OS cells were transfected with Barkor-EGFP (green) and RFP-Beclin 1 (red), and fluorescence of Barkor-EGFP (green) and RFP-Beclin 1 (red) was observed. (VII–IX) U2OS cells were transfected with RFP-Beclin 1, Myc-Barkor, and GFP-LC3, and fluorescence of GFP-LC3 (green) and RFP-Beclin 1 (red) was observed. (X–XII), U2OS cells were transfected with RFP-Beclin 1 and Barkor CCD-deletion mutant-fused EGFP, and the fluorescence of GFP-Barkor CCD deletion (green) and RFP-Beclin 1 (red) was observed.
Similar articles
- Regulation of Beclin 1 in autophagy.
Sun Q, Fan W, Zhong Q. Sun Q, et al. Autophagy. 2009 Jul;5(5):713-6. doi: 10.4161/auto.5.5.8524. Epub 2009 Jul 24. Autophagy. 2009. PMID: 19372752 Free PMC article. - Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG.
Itakura E, Kishi C, Inoue K, Mizushima N. Itakura E, et al. Mol Biol Cell. 2008 Dec;19(12):5360-72. doi: 10.1091/mbc.e08-01-0080. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843052 Free PMC article. - Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis.
Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, Pledger WJ, Wang HG. Takahashi Y, et al. Nat Cell Biol. 2007 Oct;9(10):1142-51. doi: 10.1038/ncb1634. Epub 2007 Sep 23. Nat Cell Biol. 2007. PMID: 17891140 Free PMC article. - Beclin-1: autophagic regulator and therapeutic target in cancer.
Fu LL, Cheng Y, Liu B. Fu LL, et al. Int J Biochem Cell Biol. 2013 May;45(5):921-4. doi: 10.1016/j.biocel.2013.02.007. Epub 2013 Feb 16. Int J Biochem Cell Biol. 2013. PMID: 23420005 Review. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
- Myosins, Actin and Autophagy.
Kruppa AJ, Kendrick-Jones J, Buss F. Kruppa AJ, et al. Traffic. 2016 Aug;17(8):878-90. doi: 10.1111/tra.12410. Epub 2016 May 31. Traffic. 2016. PMID: 27146966 Free PMC article. Review. - β-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy.
Liu J, Li QX, Wang XJ, Zhang C, Duan YQ, Wang ZY, Zhang Y, Yu X, Li NJ, Sun JP, Yi F. Liu J, et al. Cell Death Dis. 2016 Apr 7;7(4):e2183. doi: 10.1038/cddis.2016.89. Cell Death Dis. 2016. PMID: 27054338 Free PMC article. - Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle.
Starr T, Child R, Wehrly TD, Hansen B, Hwang S, López-Otin C, Virgin HW, Celli J. Starr T, et al. Cell Host Microbe. 2012 Jan 19;11(1):33-45. doi: 10.1016/j.chom.2011.12.002. Cell Host Microbe. 2012. PMID: 22264511 Free PMC article. - The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism.
Xiong X, Tao R, DePinho RA, Dong XC. Xiong X, et al. J Biol Chem. 2012 Nov 9;287(46):39107-14. doi: 10.1074/jbc.M112.412569. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992773 Free PMC article. - mTOR activates the VPS34-UVRAG complex to regulate autolysosomal tubulation and cell survival.
Munson MJ, Allen GF, Toth R, Campbell DG, Lucocq JM, Ganley IG. Munson MJ, et al. EMBO J. 2015 Sep 2;34(17):2272-90. doi: 10.15252/embj.201590992. Epub 2015 Jul 2. EMBO J. 2015. PMID: 26139536 Free PMC article.
References
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res. 2007;17:839–849. - PubMed
- Levine B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. - PubMed
- Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature. 1999;402:672–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials