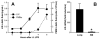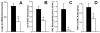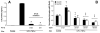Ethanol exposure impairs LPS-induced pulmonary LIX expression: alveolar epithelial cell dysfunction as a consequence of acute intoxication - PubMed (original) (raw)
Ethanol exposure impairs LPS-induced pulmonary LIX expression: alveolar epithelial cell dysfunction as a consequence of acute intoxication
James E Walker Jr et al. Alcohol Clin Exp Res. 2009 Feb.
Abstract
Background: Alcohol intoxication impairs innate immune responses to bacterial pneumonia, including neutrophil influx. Lipopolysaccharide (LPS)-induced chemokine (LIX or CXCL5) is a recently described chemokine produced by type-II alveolar epithelial (AE2) cells which facilitates neutrophil recruitment. The effect of acute alcohol intoxication on AE2 cell expression of LIX is unknown.
Methods: C57BL/6 mice were given an intraperitoneal (i.p.) injection of ethanol (4 g/kg) or saline 30 minutes prior to intratracheal (i.t.) injection with 10 mug Escherichia coli LPS. In vitro stimulation of primary AE2 cells or murine AE2 cell line MLE-12 was performed with LPS and tumor necrosis factor-alpha (TNF-alpha).
Results: LIX protein is readily detectable in the lung but not in plasma following LPS administration, demonstrating "compartmentalization" of this chemokine during pulmonary challenge. In contrast to the CXC chemokines keratinocyte-derived chemokine and macrophage inflammatory protein-2, which are abundantly expressed in both lung tissue and alveolar macrophages, LIX expression is largely confined to the lung parenchyma. Compared to controls, intoxicated animals show a decrease in LIX and neutrophil number in bronchoalveolar lavage fluid following LPS challenge. Ethanol inhibits LIX at the transcriptional level. In vitro studies show that LPS and TNF-alpha are synergistic in inducing LIX by either primary AE2 or MLE-12 cells. Acute ethanol exposure potently and dose-dependently inhibits LIX expression by AE2 cells. Activation of nuclear factor-kappaB is critical to LIX expression in MLE-12 cells, and acute ethanol treatment interferes with early activation of this pathway as evidenced by impairing phosphorylation of p65 (RelA). Inhibition of p38 mitogen-activated protein kinase signaling, but not ERK1/2 activity, in MLE-12 cells by acute alcohol is likely an important cause of decreased LIX expression during challenge.
Conclusions: These data demonstrate direct suppression of AE2 cell innate immune function by ethanol and add to our understanding of the mechanisms by which acute intoxication impairs the lung's response to microbial challenge.
Figures
Figure 1
Onset of LIX expression and neutrophil recruitment following i.t. LPS challenge (10 }g). C57BL/6 mice were sacrificed at specified time points for BAL. Alveolar LIX protein expression increases after 1 h and peaks 4 h post-challenge. Neutrophil recruitment into the alveolar space quickly increases following the peak of LIX expression (A). LIX mRNA expression (peak level 2 h post-LPS) is largely confined to lung parenchymal cells, as minimal induction is seen in alveolar macrophages (AM) (B). * p<0.05 vs. lung tissue.
Figure 2
LPS-induced LIX, TNF-αexpression and neutrophil recruitment are attenuated during acute intoxication. C57BL/6 mice were given i.p. injection of PBS (black bars) or ethanol (4.0g/kg, open bars) followed by 10 }g i.t. LPS challenge 30 min later. Ethanol intoxication inhibits both lung LIX mRNA transcription at 2 h (A) and protein expression at 4 h (B) following LPS challenge, times which represent their peaks of expression. 4 h neutrophil recruitment into alveolar space is significantly impaired during intoxication (C) as is 90 min TNF-αin BAL fluid (D). * p<0.05 vs. non-intoxicated mice.
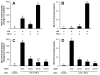
Figure 3
Primary AE2 cell and MLE-12 cell LIX expression is suppressed by acute alcohol exposure. Cells were harvested, allowed to rest overnight, and stimulated for 16 hr with LPS (500 ng/mL) or TNF-α(10 ng/mL) alone or in combination. Either stimulus alone is sufficient for LIX expression in primary AE2 cells, whereas combined stimulation results in maximal LIX expression (A). In MLE-12 cells, combination stimulation induces a robust LIX response (B). 90-min ethanol pretreatment followed by LPS/TNF-αstimulation shows that alcohol dose-dependently suppresses LIX expression in primary AE2 cells (C) and MgLE-12 cells (D). Letters indicate statistically significant differences (p<0.05).
Figure 4
LIX expression by MLE-12 cells is dependent upon NF-κB signaling. 60 min pretreatment with NF-κB inhibitors SN50 or BAY 11–7082 prior to 16-hr LPS/TNF-αstimulation markedly impairs LIX production (A). 90 min ethanol treatment prior to 16-hr LPS/TNF-αstimulation significantly reduces phosphorylation of the major trans-activating NF-κB domain p65 (RelA) at Ser468 and Ser536 residues, reflecting a substantial and ethanol dose-dependent decrease in the level of active NF-κB signaling (B). * p<0.05 vs. stimulated cells in absence of inhibitor. † p<0.05 vs. unstimulated cells. ‡ p<0.05 vs. stimulated cell without ethanol pre-treatment.
Figure 5
MAPK pathway activation is necessary for LIX expression in LPS-challenged MLE-12 cells and shows selective suppression by alcohol exposure. MLE-12 cells were pretreated for 90-min with selective p38 inhibitors (10 μM SB202190 or 0.5 μM SB203580) or ERK1/2 inhibitors (0.1 μM U0126 or 50 μM PD98059) prior to 16-hr LPS/TNF-αstimulation. MAPK blockade decreases LIX expression, with a greater effect of p38 inhibition (A). 90-min ethanol pretreatment prior to 16-hr LPS/TNF-αstimulation inhibits p38 phosphorylation/activation (B), but has no effect on ERK1/2 phosphorylation/activation (C). * p<0.05 vs. stimulated cells in absence of inhibitor or ethanol.
Similar articles
- Phospholipase Cε plays a crucial role in neutrophilic inflammation accompanying acute lung injury through augmentation of CXC chemokine production from alveolar epithelial cells.
Umezawa K, Nagano T, Kobayashi K, Dokuni R, Katsurada M, Yamamoto M, Yoshikawa Y, Kataoka T, Nishimura Y. Umezawa K, et al. Respir Res. 2019 Jan 11;20(1):9. doi: 10.1186/s12931-019-0975-4. Respir Res. 2019. PMID: 30634975 Free PMC article. - Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide.
Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Happel KI, et al. Alcohol. 2007 Aug;41(5):325-33. doi: 10.1016/j.alcohol.2007.06.002. Alcohol. 2007. PMID: 17889309 Free PMC article. - Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide.
Zhang P, Zhong Q, Bagby GJ, Nelson S. Zhang P, et al. Alcohol Clin Exp Res. 2007 Jan;31(1):113-21. doi: 10.1111/j.1530-0277.2006.00269.x. Alcohol Clin Exp Res. 2007. PMID: 17207109 - CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis.
Lin M, Carlson E, Diaconu E, Pearlman E. Lin M, et al. J Leukoc Biol. 2007 Mar;81(3):786-92. doi: 10.1189/jlb.0806502. Epub 2006 Nov 16. J Leukoc Biol. 2007. PMID: 17110418 Free PMC article. - Transcriptional profiling of lipopolysaccharide-induced acute lung injury.
Jeyaseelan S, Chu HW, Young SK, Worthen GS. Jeyaseelan S, et al. Infect Immun. 2004 Dec;72(12):7247-56. doi: 10.1128/IAI.72.12.7247-7256.2004. Infect Immun. 2004. PMID: 15557650 Free PMC article.
Cited by
- Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel.
Patel AR, Chougule MB, I T, Patlolla R, Wang G, Singh M. Patel AR, et al. Pharm Res. 2013 May;30(5):1435-46. doi: 10.1007/s11095-013-0984-9. Epub 2013 Jan 30. Pharm Res. 2013. PMID: 23361589 Free PMC article. - Advanced drug delivery to the lymphatic system: lipid-based nanoformulations.
Ali Khan A, Mudassir J, Mohtar N, Darwis Y. Ali Khan A, et al. Int J Nanomedicine. 2013;8:2733-44. doi: 10.2147/IJN.S41521. Epub 2013 Jul 26. Int J Nanomedicine. 2013. PMID: 23926431 Free PMC article. Review. - Phospholipase Cε plays a crucial role in neutrophilic inflammation accompanying acute lung injury through augmentation of CXC chemokine production from alveolar epithelial cells.
Umezawa K, Nagano T, Kobayashi K, Dokuni R, Katsurada M, Yamamoto M, Yoshikawa Y, Kataoka T, Nishimura Y. Umezawa K, et al. Respir Res. 2019 Jan 11;20(1):9. doi: 10.1186/s12931-019-0975-4. Respir Res. 2019. PMID: 30634975 Free PMC article. - Innate Immunity and Alcohol.
Kany S, Janicova A, Relja B. Kany S, et al. J Clin Med. 2019 Nov 14;8(11):1981. doi: 10.3390/jcm8111981. J Clin Med. 2019. PMID: 31739600 Free PMC article. Review. - Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients.
Depp TB, McGinnis KA, Kraemer K, Akgün KM, Edelman EJ, Fiellin DA, Butt AA, Crystal S, Gordon AJ, Freiberg M, Gibert CL, Rimland D, Bryant KJ, Crothers K. Depp TB, et al. AIDS. 2016 Jan 28;30(3):455-63. doi: 10.1097/QAD.0000000000000940. AIDS. 2016. PMID: 26765938 Free PMC article.
References
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184(9):1134–1142. - PubMed
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. - PubMed
- Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103(18):2296–2302. - PubMed
- Ci X, Song Y, Zeng F, Zhang X, Li H, Wang X, Cui J, Deng X. Ceftiofur impairs pro-inflammatory cytokine secretion through the inhibition of the activation of NF-kappaB and MAPK. Biochem Biophys Res Commun. 2008;372(1):73–77. - PubMed
- Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14(4):309–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 AA009803/AA/NIAAA NIH HHS/United States
- P60 K08 AA 15163/AA/NIAAA NIH HHS/United States
- R01 HL 091958/HL/NHLBI NIH HHS/United States
- K08 AA 15163/AA/NIAAA NIH HHS/United States
- K08 AA015163/AA/NIAAA NIH HHS/United States
- R01 HL091958/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous