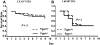Reduced thrombin generation increases host susceptibility to group A streptococcal infection - PubMed (original) (raw)
Reduced thrombin generation increases host susceptibility to group A streptococcal infection
Hongmin Sun et al. Blood. 2009.
Abstract
Bacterial plasminogen activators are commonplace among microbial pathogens, implying a central role of host plasmin in supporting bacterial virulence. Group A streptococci (GAS) secrete streptokinase, a specific activator of human plasminogen (PLG). The critical contribution of the streptokinase-PLG interaction to GAS pathogenicity was recently demonstrated using mice expressing human PLG. To examine the importance of thrombin generation in antimicrobial host defense, we challenged mice with deficiency of factor V (FV) in either the plasma or platelet compartment. Reduction of FV in either pool resulted in markedly increased mortality after GAS infection, with comparison to heterozygous F5-deficient mice suggesting a previously unappreciated role for the platelet FV pool in host defense. Mice with complete deficiency of fibrinogen also demonstrated markedly increased mortality to GAS infection relative to controls. Although FV Leiden may be protective in the setting of severe sepsis in humans, no significant survival advantage was observed in GAS-infected mice carrying the FV Leiden mutation. Taken together, our data support the hypothesis that local thrombosis/fibrin deposition limits the survival and dissemination of at least a subset of microbial pathogens and suggest that common variation in hemostatic factors among humans could affect host susceptibility to a variety of infectious diseases.
Figures
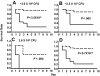
Figure 1
Control mice of the FV-transgenic lines inoculated with GAS strain UMAA2616. F5+/−, Tg+F5+/−, Tg+F5+/+, and F5+/+ mice for all 3 FV-transgenic lines were infected with UMAA2616 at doses of 3.6 × 106 to 2.6 × 108 CFU. Data were pooled from 7 independent experiments using littermates, constituting a total of 18 F5+/−, 27 Tg+F5+/−, 3 Tg+F5+/+, and 7 F5+/+ mice.
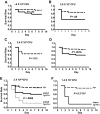
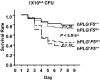
Figure 2
AlbfvB mice inoculated with GAS strain UMAA2616. P values for differences between the survival curves are indicated in all panels. The solid line represents Tg+_F5_−/−; dashed lines, F5+ littermate controls. (A) Inoculum of 3.6 × 106 CFU. Data were pooled from 4 independent experiments (totaling 17 AlbfvBTg+_F5_−/− mice and 32 littermate F5+ controls), at doses ranging from 1.2 × 106 to 4.9 × 106 CFU. (B) Inoculum of 2.5 × 107 CFU. Data were pooled from 2 independent experiments (totaling 6 AlbfvBTg+_F5_−/− mice and 18 littermate F5+ controls) at doses of 1.4 × 107 and 3.6 × 107 CFU. (C) Inoculum of 2.6 × 108 CFU. Data were from a single experiment with 5 AlbfvBTg+_F5_−/− mice and 5 F5+ littermate controls. (D) Pooled data from all the experiments shown in panels A to C (a total of 28 AlbfvBTg+_F5_−/− mice and 55 F5+ littermate controls).
Figure 3
AlbfvC and Pf4fvA mice inoculated with GAS strain UMAA2616. P values for survival differences are indicated in each panel. — represents Tg+_F5_−/−; ----, F5+ littermate controls. (A) AlbfvC mice at an inoculum of approximately 4 × 106 CFU. Data were pooled from 2 independent experiments (totaling 17 AlbfvCTg+_F5_−/− mice and 32 littermate F5+ controls), at doses of 3.7 × 106 and 4.4 × 106 CFU, respectively. (B) AlbfvC mice at an inoculum of 3.6 × 107 CFU. Data were derived from 1 experiment (4 AlbfvCTg+_F5_−/− mice and 22 littermate F5+ controls). (C) AlbfvC mice at an inoculum of 2.6 × 108 CFU. Data were derived from 1 experiment (4 AlbfvCTg+_F5_−/− mice and 9 littermate F5+controls). (D) Pooled data from all experiments (25 AlbfvCTg+_F5_−/− mice and 63 littermate F5+ controls). (E) Comparison of the AlbfvC and AlbfvB lines (combined from Figures 3D and 2D). P value of the difference between Tg+_F5_−/− mice from AlbfvC and AlbfvB lines is indicated. (F) Pf4fvA mice at an inoculum of 1.4 × 107 CFU. Data were derived from 1 experiment (6 Pf4fvATg+_F5_−/− mice and 25 littermate F5+ controls).
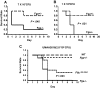
Figure 4
_Fga_−/− and Fga+/− mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents _Fga_−/−; ----, Fga+/− littermate controls. (A) Five _Fga_−/− mice and 5 sibling control Fga+/− mice were infected with 7 × 107 CFU UMAA2616. (B) Five _Fga_−/− mice and 5 sibling control Fga+/− mice were infected with 106 CFU UMAA2616. (C) Ten _Fib_γ_390_-396A and wild-type sibling controls, 9 _Fga_−/−, and 10 Fga+/− sibling control were infected with 2.8 × 106 CFU UMAA2616.
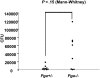
Figure 5
GAS systemic spread in _Fga_−/− and Fga+/− mice infected with UMAA2616. Bacterial counts from spleens of 8 _Fga_−/− and 9 Fga+/− mice inoculated with 2 × 106 CFU UMAA2616 were calculated by a plating method. Data were pooled from 2 independent experiments (2.8 × 106 and 2.0 × 106 CFU). The difference of CFU between _Fga_−/− and Fga+/− mice was tested by the Mann-Whitney test. P value is indicated.
Figure 6
Fga+/− and Fga+/+ mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents Fga+/−; ----, Fga+/+ littermate controls. (A) Fga+/− mice and sibling control Fga+/+ mice were infected with 2 × 107 to 108 CFU UMAA2616. Data were pooled from 4 independent experiments totaling 38 Fga+/− mice and 33 Fga+/+ mice. (B) Fga+/− mice and sibling control Fga+/+ mice were inoculated with 1.4 × 109 CFU UMAA2616, totaling 17 Fga+/− mice and 22 Fga+/+ mice in 1 experiment.
Figure 7
FV Leiden mice inoculated with GAS strain UMAA2616. Data were pooled from 11 independent experiments with UMAA2616 doses ranging from 106 to 108 CFU. The total number of mice in each group are 68 hPLG_−_F5+/+, 74 hPLG+F5+/+, 61 hPLG_−_F5 Q/+, and 57 hPLG+F5 Q/+. P values for survival difference are indicated. Among the P values indicated are difference between hPLG_−_F5+/+ and hPLG_−_F5 Q/+ (P > .26), difference between combined _hPLG_− (hPLG_−_F5+/+ and hPLG_−_F5 Q/+) and combined hPLG+ (hPLG+F5+/+ and hPLG+F5 Q/+) (P < 5.5 × 10−9), and difference between _hPLG_+_F5_ _Q_/+ and _hPLG_+_F5_+/+ (_P_ > .52).
Similar articles
- Plasminogen is a critical host pathogenicity factor for group A streptococcal infection.
Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjöbring U, Ginsburg D. Sun H, et al. Science. 2004 Aug 27;305(5688):1283-6. doi: 10.1126/science.1101245. Science. 2004. PMID: 15333838 - A key role for the urokinase plasminogen activator (uPA) in invasive Group A streptococcal infection.
Sanderson-Smith ML, Zhang Y, Ly D, Donahue D, Hollands A, Nizet V, Ranson M, Ploplis VA, Walker MJ, Castellino FJ. Sanderson-Smith ML, et al. PLoS Pathog. 2013;9(7):e1003469. doi: 10.1371/journal.ppat.1003469. Epub 2013 Jul 4. PLoS Pathog. 2013. PMID: 23853591 Free PMC article. - Plasminogen enhances virulence of group A streptococci by streptokinase-dependent and streptokinase-independent mechanisms.
Khil J, Im M, Heath A, Ringdahl U, Mundada L, Cary Engleberg N, Fay WP. Khil J, et al. J Infect Dis. 2003 Aug 15;188(4):497-505. doi: 10.1086/377100. Epub 2003 Aug 5. J Infect Dis. 2003. PMID: 12898435 - The role of streptokinase as a virulence determinant of Streptococcus pyogenes--potential for therapeutic targeting.
McArthur JD, Cook SM, Venturini C, Walker MJ. McArthur JD, et al. Curr Drug Targets. 2012 Mar;13(3):297-307. doi: 10.2174/138945012799424589. Curr Drug Targets. 2012. PMID: 22206253 Review. - Uncovering the mysteries of invasive streptococcal diseases.
Chhatwal GS, McMillan DJ. Chhatwal GS, et al. Trends Mol Med. 2005 Apr;11(4):152-5. doi: 10.1016/j.molmed.2005.02.001. Trends Mol Med. 2005. PMID: 15823752 Review.
Cited by
- The effect of heparin administration in animal models of sepsis: a prospective study in Escherichia coli-challenged mice and a systematic review and metaregression analysis of published studies.
Li Y, Sun JF, Cui X, Mani H, Danner RL, Li X, Su JW, Fitz Y, Eichacker PQ. Li Y, et al. Crit Care Med. 2011 May;39(5):1104-12. doi: 10.1097/CCM.0b013e31820eb718. Crit Care Med. 2011. PMID: 21317646 Free PMC article. Review. - Relevant role of von Willebrand factor in neutrophil recruitment in a mouse sepsis model involving cecal ligation and puncture.
Kasuda S, Matsui H, Ono S, Matsunari Y, Nishio K, Shima M, Hatake K, Sugimoto M. Kasuda S, et al. Haematologica. 2016 Feb;101(2):e52-4. doi: 10.3324/haematol.2015.135434. Epub 2015 Oct 22. Haematologica. 2016. PMID: 26494840 Free PMC article. No abstract available. - COVID-19 and Sepsis Are Associated With Different Abnormalities in Plasma Procoagulant and Fibrinolytic Activity.
Bouck EG, Denorme F, Holle LA, Middelton EA, Blair AM, de Laat B, Schiffman JD, Yost CC, Rondina MT, Wolberg AS, Campbell RA. Bouck EG, et al. Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):401-414. doi: 10.1161/ATVBAHA.120.315338. Epub 2020 Nov 16. Arterioscler Thromb Vasc Biol. 2021. PMID: 33196292 Free PMC article. - Platelet-Derived Factor V Is an Important Determinant of the Metastatic Potential of Circulating Tumor Cells.
Deng X, Feng Z, Zhu L, Chen N, Deng Y, Li Y, Li R, Wang L, Luo M, Wu J. Deng X, et al. Front Oncol. 2020 Sep 23;10:558306. doi: 10.3389/fonc.2020.558306. eCollection 2020. Front Oncol. 2020. PMID: 33072582 Free PMC article. - Disease manifestations and pathogenic mechanisms of Group A Streptococcus.
Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. Walker MJ, et al. Clin Microbiol Rev. 2014 Apr;27(2):264-301. doi: 10.1128/CMR.00101-13. Clin Microbiol Rev. 2014. PMID: 24696436 Free PMC article. Review.
References
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. - PubMed
- Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. - PubMed
- Bisno AL, Rubin FA, Cleary PP, Dale JB. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles—report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis. 2005;41:1150–1156. - PubMed
- Harrington DJ, Sutcliffe IC, Chanter N. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 2002;4:501–510. - PubMed
- Marcum JA, Kline DL. Species specificity of streptokinase. Comp Biochem Physiol B. 1983;75:389–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- R21AI076675-01/AI/NIAID NIH HHS/United States
- R37HL039693/HL/NHLBI NIH HHS/United States
- R01 HL085357/HL/NHLBI NIH HHS/United States
- R01HL85357/HL/NHLBI NIH HHS/United States
- R21 AI076675/AI/NIAID NIH HHS/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- P01HL057346/HL/NHLBI NIH HHS/United States
- R37 HL039693/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous