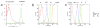A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester - PubMed (original) (raw)
A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester
Michael Miksa et al. J Immunol Methods. 2009.
Abstract
Apoptotic cell phagocytosis has recently raised considerable interest, particularly due to its intricate molecular mechanisms and negative immunologic impact of incompetent clearance of apoptotic cells. There is a need for simple and reliable methods to clearly determine the internalization of apoptotic cells. Labeling with pHrodo succinimidyl ester (SE), a pH-sensitive fluorescent dye, makes engulfed apoptotic cells detectable due to the increased post-phagocytic light emission. This is a valuable tool for phagocytosis studies via FACS. We designed an ex vivo assay, using apoptotic pHrodo-labeled lymphocytes as prey and anti-CD11b-labeled tissue macrophages. To demonstrate its validity of detecting internalized apoptotic lymphocytes, we used MFGE8(-/-) macrophages, known to have impaired phagocytic ability. Uptake of apoptotic lymphocytes was accelerated and enhanced in splenic macrophages after stimulation with recombinant MFGE8, while peritoneal macrophages were able to compensate for the delayed uptake. This novel assay is a quick and reliable method to evaluate the internalization of apoptotic cells.
Figures
Figure 1
pHrodo-SE labeling of apoptotic cells and responsiveness to changes in pH. (a) Annexin V staining of non-treated (non-apoptotic) and dexamethasone-treated (apoptotic, 0.1 μM for 16h) rat thymocytes, as analyzed by flow cytometry. (b) Dose-dependent pHrodo-SE staining of apoptotic cells. 107 apoptotic thymocytes were stained with 0.01-1 μg/ml pHrodo-SE, as analyzed by flow cytometry. (c) Responsiveness of pHrodo-SE-labeled apoptotic thymocytes to changes in pH. pHrodo-SE-labeled cells (0.01 μg/ml) were exposed to a pH of 7.4, 4.0 and 3.0 by adding HCl to the cell suspension, as analyzed by flow cytometry. Mean fluorescent intensity (MFI) is shown next to FACS plot.
Figure 2
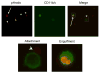
Fluorescent intensity of pHrodo-SE-labeled apoptotic thymocytes increases after engulfment by macrophages. Splenic macrophages were labeled with FITC-anti-CD11b/c (OX42) and thymocytes with pHrodo-SE (0.02 μg/ml). Cells were coincubated for 60 min, collected and fixed with 1%PFA prior to fluorescent microscopy. Top three panels show lymphocytes in red (pHrodo-SE+), macrophages in green (CD11b/c) and a merged image, of the same area to the right. Boxes in the merged image indicate the 10× magnification shown below. Arrowheads indicate free floating and attached apoptotic thymocytes and arrows indicate engulfed apoptotic cells. Magnification: 400×.
Figure 3
Effect of pHrodo-SE on phagocytosis. Thymocytes were collected from normal rats and incubated with (apoptotic) or without (non-apoptotic) 0.1μM of dexamethasone for 16h. Cells were labeled with pHrodo-SE, cocultured with peritoneal macrophages for 60min, then stained with FITC-anti-CD11b/c and analyzed by flow cytometry immediately. Representative phagocytosis result shown of CD11b/c and pHrodo-SE (gated on CD11b/c+) of (a) non-apoptotic thymocytes, and (b) apoptotic thymocytes. (c) Average percent phagocytosis index ± SEM of CD11b/c+pHrodo+ cells. (d) Average mean fluorescent intensity (MFI) ± SEM of CD11b/c+pHrodo+ cells. *P<0.05 vs. non-apoptotic, Student's _t_-test, _n_=3.
Figure 4
Verification of deficient phagocytosis of apoptotic cells by MFGE8-deficient splenic macrophages. (a) Representative FACS histograms. MFGE8-deficient splenic CD11b+ macrophages were incubated with autologous pHrodo-labeled thymocytes (0.02 μg/ml*106 cells) for 5 to 180 minutes and analyzed by flow cytometry in 3days. (b) Line graph showing means ± SEM (percent phagocytosing macrophages) of triplicates of a representative experiment. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, _n_=3. (c) Average mean fluorescent intensity (MFI) and SEM of CD11b+pHrodo-SE+ cells in one representative experiment performed in triplicate. This represents an estimate for the amount of phagocytized cells by the macrophages. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, _n_=3.
Figure 5
MFGE8-deficient resident peritoneal macrophages compensate for a delay in apoptotic cell clearance. MFGE8-deficient peritoneal CD11b+ macrophages were incubated with autologous pHrodo-SE-labeled thymocytes (0.02 μg/ml*106 cells) for 5 to 180 minutes and analyzed by flow cytometry. Means ± SEM (percent phagocytosing macrophages) of triplicates of a representative experiment. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, _n_=3.
Figure 6
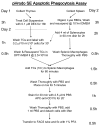
Flow chart of pHrodo-SE-staining and phagocytosis assay protocol.
Similar articles
- Measurement of phagocytic engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester.
Aziz M, Yang WL, Wang P. Aziz M, et al. Curr Protoc Immunol. 2013;Chapter 14:Unit 14.31.. doi: 10.1002/0471142735.im1431s100. Curr Protoc Immunol. 2013. PMID: 23392636 - Efferocytosis assay to quantify the engulfment and acidification of apoptotic cells by macrophages using flow cytometry.
Wu X, Wang Z, Shern T, Zhang H. Wu X, et al. STAR Protoc. 2024 Sep 20;5(3):103215. doi: 10.1016/j.xpro.2024.103215. Epub 2024 Jul 27. STAR Protoc. 2024. PMID: 39068649 Free PMC article. - Polymicrobial sepsis enhances clearance of apoptotic immune cells by splenic macrophages.
Swan R, Chung CS, Albina J, Cioffi W, Perl M, Ayala A. Swan R, et al. Surgery. 2007 Aug;142(2):253-61. doi: 10.1016/j.surg.2007.04.005. Surgery. 2007. PMID: 17689693 Free PMC article. - An optimized protocol to determine the engulfment of cancer cells by phagocytes using flow cytometry and fluorescence microscopy.
Nam GH, Hong Y, Choi Y, Kim GB, Kim YK, Yang Y, Kim IS. Nam GH, et al. J Immunol Methods. 2019 Jul;470:27-32. doi: 10.1016/j.jim.2019.04.007. Epub 2019 Apr 26. J Immunol Methods. 2019. PMID: 31034881 - [In vivo tracing of transferred apoptotic cell labeled using CFSE: a flow cytometry-based assay method].
Wang Y, Gao Y, Sun EW, Xie JM, Zhang HY, Chen JB. Wang Y, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2006 May;26(5):599-602. Nan Fang Yi Ke Da Xue Xue Bao. 2006. PMID: 16762859 Chinese.
Cited by
- Protocol for monitoring phagocytosis of cancer cells by TAM-like macrophages using imaging cytometry.
Mishra AK, Banday S, Thakare RP, Malonia SK, Green MR. Mishra AK, et al. STAR Protoc. 2024 Sep 18;5(4):103320. doi: 10.1016/j.xpro.2024.103320. Online ahead of print. STAR Protoc. 2024. PMID: 39298319 Free PMC article. - Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes.
Johns JL, Baumgartner TR, Sanchez CR, Dolan BP. Johns JL, et al. Animals (Basel). 2024 Aug 7;14(16):2297. doi: 10.3390/ani14162297. Animals (Basel). 2024. PMID: 39199831 Free PMC article. - The Role of TIM-1 and CD300a in Zika Virus Infection Investigated with Cell-Based Electrical Impedance.
Oeyen M, Heymann CJF, Jacquemyn M, Daelemans D, Schols D. Oeyen M, et al. Biosensors (Basel). 2024 Jul 25;14(8):362. doi: 10.3390/bios14080362. Biosensors (Basel). 2024. PMID: 39194591 Free PMC article. - ACK1 and BRK non-receptor tyrosine kinase deficiencies are associated with familial systemic lupus and involved in efferocytosis.
Guillet S, Lazarov T, Jordan N, Boisson B, Tello M, Craddock B, Zhou T, Nishi C, Bareja R, Yang H, Rieux-Laucat F, Lorenzo RIF, Dyall SD, Isenberg D, D'Cruz D, Lachmann N, Elemento O, Viale A, Socci ND, Abel L, Nagata S, Huse M, Miller WT, Casanova JL, Geissmann F. Guillet S, et al. medRxiv [Preprint]. 2024 Jun 5:2024.02.15.24302255. doi: 10.1101/2024.02.15.24302255. medRxiv. 2024. PMID: 38883731 Free PMC article. Preprint. - epHero - a tandem-fluorescent probe to track the fate of apoptotic cells during efferocytosis.
Singh S, Bensalem J, Hein LK, Casey A, Mäkinen VP, Sargeant TJ. Singh S, et al. Cell Death Discov. 2024 Apr 17;10(1):179. doi: 10.1038/s41420-024-01952-1. Cell Death Discov. 2024. PMID: 38632247 Free PMC article.
References
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77. - PubMed
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8. - PubMed
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG028352/AG/NIA NIH HHS/United States
- R01 GM053008/GM/NIGMS NIH HHS/United States
- R01 GM053008-15/GM/NIGMS NIH HHS/United States
- R01 GM057468-12/GM/NIGMS NIH HHS/United States
- R01 AG028352-03/AG/NIA NIH HHS/United States
- R01 GM057468/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous