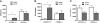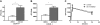Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization - PubMed (original) (raw)
Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization
Matthew J Christopher et al. Blood. 2009.
Abstract
Current evidence suggests that hematopoietic stem/progenitor cell (HSPC) mobilization by granulocyte colony-stimulating factor (G-CSF) is mediated by induction of bone marrow proteases, attenuation of adhesion molecule function, and disruption of CXCL12/CXCR4 signaling in the bone marrow. The relative importance and extent to which these pathways overlap or function independently are uncertain. Despite evidence of protease activation in the bone marrow, HSPC mobilization by G-CSF or the chemokine Grobeta was abrogated in CXCR4(-/-) bone marrow chimeras. In contrast, HSPC mobilization by a VLA-4 antagonist was intact. To determine whether other mobilizing cytokines disrupt CXCR4 signaling, we characterized CXCR4 and CXCL12 expression after HSPC mobilization with Flt3 ligand (Flt3L) and stem cell factor (SCF). Indeed, treatment with Flt3L or SCF resulted in a marked decrease in CXCL12 expression in the bone marrow and a loss of surface expression of CXCR4 on HSPCs. RNA in situ and sorting experiments suggested that the decreased CXCL12 expression is secondary to a loss of osteoblast lineage cells. Collectively, these data suggest that disruption of CXCR4 signaling and attenuation of VLA-4 function are independent mechanisms of mobilization by G-CSF. Loss of CXCL12 expression by osteoblast appears to be a common and key step in cytokine-induced mobilization.
Figures
Figure 1
G-CSF treatment does not increase the number of circulating progenitors in _CXCR4_−/− chimeras. Wild-type (CXCR4+/+) and CXCR4−/− chimeras (n = 7-10 each group) were treated with G-CSF or left untreated (control), and CFU-Cs were measured in (A) peripheral blood, (B) spleen, and (C) bone marrow. Data represent mean ± SEM of pooled data from 2 separate experiments; *P < .01.
Figure 2
Bone marrow metalloproteinases and neutrophil elastase are induced normally in _CXCR4_−/− chimeras. Bone marrow chimeras (n = 2-4 each group) reconstituted with wild-type (CXCR4+/+) or _CXCR4_−/− fetal liver cells were treated with G-CSF or left untreated (ctrl) and bone marrow plasma was isolated. Metalloproteinase (A) and neutrophil elastase (B) activity in the bone marrow plasma was estimated by measuring cleavage of labeled substrate and normalizing for protein content. Data represent mean ± SEM; *P < .05; **P < .01.
Figure 3
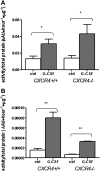
VLA-4 antagonism increases number of circulating HSPCs in CXCR4+/+ and _CXCR4_−/− chimeras. CXCR4+/+ and _CXCR4_−/− chimeras (n = 6-9 each group) were treated with BIO5192, a specific VLA-4 antagonist, and peripheral blood CFU-Cs were measured in (A) CXCR4+/+ and (B) _CXCR4_−/− chimeras. (C) Recipient mice (n = 3 each group) were administered VLA-4 inhibitor or vehicle 3 hours before adoptive transfer of 6 × 106 wild-type bone marrow mononuclear cells. The number of circulating CFU-Cs was measured before adoptive transfer (t = 0) and 2 and 30 minutes after adoptive transfer. Data represent mean ± SEM; *P < .01.
Figure 4
Groβ administration decreases the number of circulating HSPCs in _CXCR4_−/− chimeras. Circulating CFU-Cs were measured in CXCR4+/+ (A, n = 3-6) and _CXCR4_−/− (B, n = 5-11) chimeras at the indicated time points after administration of Groβ (2.5 mg/kg). (C) CXCR4+/+ (WT) or _CXCR4_−/− (KO) chimeras (n = 3-4 each group) were treated with Groβ or left untreated (ctrl). Serum was collected 15 minutes after treatment and assayed for metalloproteinase activity. (D) Wild-type mice (n = 4 each group) were given a single injection of AMD3100 (5 mg/kg) and 3 hours later Groβ was administered. Shown is number of circulating CFU-Cs before and 15 minutes after Groβ administration. Data represent mean ± SEM; *P < .05 compared with pretreatment; **P < .01 vs control.
Figure 5
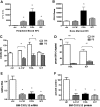
Disruption of CXCL12/CXCR4 signaling is a common feature in cytokine-induced mobilization. Wild-type mice (n = 6-8 each group) were left untreated (ctrl) or treated with G-CSF, Flt3L, or SCF for 7 days. Shown is the number of CFU-Cs in the blood (A) and bone marrow (B). (C) CXCR4 surface expression of Kit+ lineage− cells in the blood (PB) and bone marrow (BM) was measured by flow cytometry. Shown is the mean fluorescent intensity (MFI). (D) The migration of CFU-Cs isolated from the blood or bone marrow in response to CXCL12 was measured using a transwell assay. Shown is the percentage of input CFU-Cs that migrated in response to CXCL12. (E) CXCL12 mRNA expression in the bone marrow (relative to β-actin) was measured by real-time RT-PCR. (F) CXCL12 protein expression in bone marrow plasma was measured by ELISA. Shown is the expression relative to control bone marrow. Data represent mean ± SEM; *P < .05; **P < .01.
Figure 6
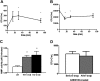
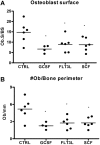
Loss of bone marrow osteoblasts is associated with cytokine-induced mobilization. Mice (n = 4-6 each group) were treated with G-CSF, Flt3L, or SCF for 7 days. Bone marrow osteoblasts were enumerated in H&E-stained paraffin sections using standard histomorphometric technique. Shown is the percentage of osteoblast-covered bone surface (A) and number of osteoblasts per bone perimeter (B). Data represent mean ± SEM; *P < .05.
Figure 7
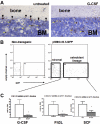
Cytokine-induced mobilization results in the specific loss of osteoblast CXCL12 expression. (A) Representative photomicrograph of CXCL12 RNA in situ from G-CSF–treated (left) and untreated (right) mouse long bones. Specific CXCL12 signal (red) was detected along endosteal surfaces (arrows) and within the bone marrow (arrowheads; n = 3-5, each group). (B) Transgenic (pOBCol2.3-GFP) mice expressing GFP in osteoblast lineage cells were treated with cytokines(n = 4-5 each group), and stromal cells were isolated and fractionated by flow cytometry into nonosteoblast and osteoblast (GFP+) fractions. Shown are representative dot plots showing the sorting strategy; data are gated on CD45− Ter119− stromal cells. (C) CXCL12 mRNA expression (relative to β-actin) in the GFP+ (osteoblast) cell fraction is shown. Data represent mean ± SEM; *P < .05.
Comment in
- HSC mobilization: new incites and insights.
Ross J, Li L. Ross J, et al. Blood. 2009 Aug 13;114(7):1283-4. doi: 10.1182/blood-2009-02-203240. Blood. 2009. PMID: 19679695 No abstract available.
Similar articles
- Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice.
Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Christopher MJ, et al. J Exp Med. 2011 Feb 14;208(2):251-60. doi: 10.1084/jem.20101700. Epub 2011 Jan 31. J Exp Med. 2011. PMID: 21282380 Free PMC article. - Downregulation of CXCL12 signaling and altered hematopoietic stem and progenitor cell trafficking in a murine model of acute Anaplasma phagocytophilum infection.
Johns JL, Borjesson DL. Johns JL, et al. Innate Immun. 2012 Jun;18(3):418-28. doi: 10.1177/1753425911413794. Epub 2011 Sep 29. Innate Immun. 2012. PMID: 21964802 Free PMC article. - Adrenaline administration promotes the efficiency of granulocyte colony stimulating factor-mediated hematopoietic stem and progenitor cell mobilization in mice.
Chen C, Cao J, Song X, Zeng L, Li Z, Li Y, Xu K. Chen C, et al. Int J Hematol. 2013 Jan;97(1):50-7. doi: 10.1007/s12185-012-1228-1. Epub 2012 Dec 8. Int J Hematol. 2013. PMID: 23224606 - Development of VLA4 and CXCR4 Antagonists for the Mobilization of Hematopoietic Stem and Progenitor Cells.
Ruminski PG, Rettig MP, DiPersio JF. Ruminski PG, et al. Biomolecules. 2024 Aug 14;14(8):1003. doi: 10.3390/biom14081003. Biomolecules. 2024. PMID: 39199390 Free PMC article. Review. - Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4.
Rettig MP, Ansstas G, DiPersio JF. Rettig MP, et al. Leukemia. 2012 Jan;26(1):34-53. doi: 10.1038/leu.2011.197. Epub 2011 Sep 2. Leukemia. 2012. PMID: 21886173 Free PMC article. Review.
Cited by
- Deregulation of the CXCL12/CXCR4 axis in methotrexate chemotherapy-induced damage and recovery of the bone marrow microenvironment.
Georgiou KR, Scherer MA, King TJ, Foster BK, Xian CJ. Georgiou KR, et al. Int J Exp Pathol. 2012 Apr;93(2):104-14. doi: 10.1111/j.1365-2613.2011.00800.x. Epub 2012 Jan 5. Int J Exp Pathol. 2012. PMID: 22220905 Free PMC article. - Pharmacological inhibition of EGFR signaling enhances G-CSF-induced hematopoietic stem cell mobilization.
Ryan MA, Nattamai KJ, Xing E, Schleimer D, Daria D, Sengupta A, Köhler A, Liu W, Gunzer M, Jansen M, Ratner N, Le Cras TD, Waterstrat A, Van Zant G, Cancelas JA, Zheng Y, Geiger H. Ryan MA, et al. Nat Med. 2010 Oct;16(10):1141-6. doi: 10.1038/nm.2217. Epub 2010 Sep 26. Nat Med. 2010. PMID: 20871610 Free PMC article. - The cardiac wound healing response to myocardial infarction.
Chalise U, Becirovic-Agic M, Lindsey ML. Chalise U, et al. WIREs Mech Dis. 2023 Jan;15(1):e1584. doi: 10.1002/wsbm.1584. Epub 2022 Oct 23. WIREs Mech Dis. 2023. PMID: 36634913 Free PMC article. Review. - Neutrophils instruct homeostatic and pathological states in naive tissues.
Casanova-Acebes M, Nicolás-Ávila JA, Li JL, García-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, A-González N, Ballesteros I, Devi S, Quintana JA, Crainiciuc G, Leiva M, Gunzer M, Weber C, Nagasawa T, Soehnlein O, Merad M, Mortha A, Ng LG, Peinado H, Hidalgo A. Casanova-Acebes M, et al. J Exp Med. 2018 Nov 5;215(11):2778-2795. doi: 10.1084/jem.20181468. Epub 2018 Oct 3. J Exp Med. 2018. PMID: 30282719 Free PMC article. - FLT3 ligand regulates thymic precursor cells and hematopoietic stem cells through interactions with CXCR4 and the marrow niche.
Williams KM, Moore AR, Lucas PJ, Wang J, Bare CV, Gress RE. Williams KM, et al. Exp Hematol. 2017 Aug;52:40-49. doi: 10.1016/j.exphem.2017.05.005. Epub 2017 May 26. Exp Hematol. 2017. PMID: 28552733 Free PMC article.
References
- Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A. Current trends in hematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374–2386. - PubMed
- Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–588. - PubMed
- Levesque JP, Winkler IG, Larsen SR, Rasko JE. Mobilization of bone marrow-derived progenitors. Handb Exp Pharmacol. 2007;180:3–36. - PubMed
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. - PubMed
- Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous