Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein - PubMed (original) (raw)
Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein
Li-Fan Lu et al. Immunity. 2009.
Abstract
Foxp3(+) regulatory T (Treg) cells limit pathogenic immune responses to self-antigens and foreign antigens. An essential role for microRNA (miRNA) in the maintenance and function of Treg cells, revealed by the Treg cell-specific Dicer ablation, raised a question as to a specific miRNA contribution. We found that Foxp3 controlled the elevated miR155 expression required for maintaining Treg cell proliferative activity and numbers under nonlymphopenic conditions. Moreover, miR155 deficiency in Treg cells resulted in increased suppressor of cytokine signaling 1 (SOCS1) expression accompanied by impaired activation of signal transducer and activator of transcription 5 (STAT5) transcription factor in response to limiting amounts of interleukin-2. Our studies suggest that Foxp3-dependent regulation of miR155 maintains competitive fitness of Treg cell subsets by targeting SOCS1, and they provide experimental support for a proposed role for miRNAs in ensuring the robustness of cellular phenotypes.
Figures
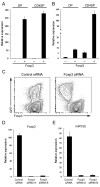
Figure 1. High amounts of miR155 expression in TR cells are driven by Foxp3
Foxp3+ and Foxp3- DP and CDSP thymocyte subsets were isolated and A, Foxp3 and B, miR155 amounts were assessed by real-time PCR. Data are representative of two independent experiments. C, Diminished amounts of miR155 in TR cells upon Foxp3 knockdown with a Foxp3-specific shRNA. Foxp3 shRNA and the corresponding scrambled shRNA (control) were expressed in TR cells using a retroviral vector equipped with a GFP reporter. D and E, GFP+ cells were sorted and the expression of Foxp3 and miR155 were measured by real-time PCR analysis 3 days after retroviral infection.
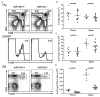
Figure 2. Reduced thymic and peripheral TR cell subsets in the absence of miR155
A, Flow cytometric analysis of Foxp3+TR cell subsets in 6-8 wk-old miR155-deficient mice and wild-type littermates. The proportions of different thymocyte subsets and of Foxp3+ cells within in CD4 SP thymocyte and CD4+ splenic T cell subsets are shown. B-D, Cellularity of the thymus and spleen, and the proportion and absolute numbers of thymic and splenic Foxp3+ and Foxp3-CD4+ T cells in miR155-deficient and -sufficient mice are shown.
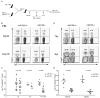
Figure 3. miR155-deficient TR cells exhibit impaired homeostais
A, Schematic of generation of mixed BM chimeric mice. B, TR cell frequencies within each donor-derived populations of peripheral blood lymphocytes at various time points after BM transfer. C, The ratios of Ly5.1-Foxp3+ (miR155-/- or WT littermates) and Ly5.1+Foxp3+ cells were plotted over time. D, TR cell frequencies within each donor derived T cell population from both the thymus and spleen 120 days after BM transfer. E, The ratio of thymic and splenic Ly5.1-Foxp3+and Ly5.1+Foxp3+cells 120 days after BM transfer. The data in C and E represent three independent experiments.
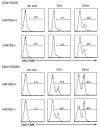
Figure 4. miR155 deficiency does not affect TR cell susceptibility to activation induced cell death
Sorted CD4+CD25- (TE) and CD4+CD25hi (TR) cells from miR155-deficient mice or littermate controls were cultured in vitro with plate-bound CD3 (1μg/ml) antibodies. Apoptotic cells were labeled with the active caspase 3 probe FITC-VAD-FMK at indicated time points and enumerated using flow cytometric analysis. Data are representative of two independent experiments (n=4).
Figure 5. Diminished proliferative activity of TR cell in the absence of miR155 in the competitive environment of lympho-replete, but not lymphopenic mice
A, Expression of Ki67 in Ly5.1+ (C57/B6) or Ly5.1- (miR155-/- or WT littermates) SP thymocytes 120 days after BM reconstitution. The frequency of Ki67+ cells in either Foxp3+ or Foxp3- population is indicated. B, The ratios of Ly5.1-Ki67+ (miR155-/- or WT littermates) and Ly5.1+Ki67+ cells within either Foxp3+ or Foxp3- CD4SP thymocyte subset. C, Expression of Ki67 in Ly5.1+ or Ly5.1- splenic CD4+ T cells. The frequency of Ki67+ cells within either Foxp3+ or Foxp3- CD4 T cell subsets is indicated. D, The ratios of Ly5.1-Ki67+ and Ly5.1+Ki67+ cells within Foxp3+ and Foxp3- splenic CD4+ T cell subsets. Data are representative of two independent experiments. E, Expression of Ki67 within each donor-derived population of peripheral blood lymphocytes at various time points after BM transfer. The ratios of Ki67+Ly5.1-Foxp3+ (miR155-/- or WT littermates) and Ki67+Ly5.1+Foxp3+ cells were analyzed over time. F, Ly5.1-T cells (miR155-/- or WT littermates) isolated from mixed BM chimeric mice 120 days after BM reconstitution were re-transferred into _RAG_-deficient mice. G, Expression of Ki67 within Foxp3-CD4+ and Foxp3+CD4+ T cell subsets from miR155-/- (black line) or WT littermates (tinted) 14 days after transfer were assessed by flow cytometric analysis. H, The frequencies of Ki67+ cells in either miR155-sufficient or -deficient population were plotted. Data are representative of two independent experiments.
Figure 6. miR155 modulates sensitivity of TR cells to IL-2 through targeting SOCS1
A, Flow cytometric analysis of Stat5 phosphorylation in miR155-deficient and -sufficient TR cells induced upon stimulation with IL-2 at the indicated concentrations. B, Expression of the SOCS1 transcript was measured by real-time PCR. TN: naïve CD4+CD25-CD62Lhi cells; TE: non-TR cells activated with anti-CD3/anti-CD28 for 48hrs; TR: CD4+CD25+ cells. C, Western blot analysis of the SOCS1 protein expression. Densitometric SOCS1 expression values normalized based on β-actin expression values are indicated below the corresponding lanes as well as fold increase in normalized SOCS1 expression in the absence of miR155 in the indicated T cell subsets. D, Multiple species sequence alignment of the SOCS1 3′ UTR including the predicted miR155 target site sequence (in bold). Mutation of the miR155 target site sequence is shown below. E, 293T cells were co-transfected with WT or mutated SOCS1 3′UTR and miR155 and assessed for luciferase activity 24hrs after transfection. MiR150 was used as a control miRNA in these experiments. F, The proportion and G, absolute numbers of thymic Foxp3+ TR cells in WT littermate control mice, SOCS1 Tg mice as well as SOCS1 cKO mice. The data shown in every panel are representative of two or more independent experiments (n=3∼6); values represent the mean +/- s.d., *P<0.005.
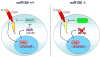
Figure 7. A model for miR155 function in TR cell homeostasis
In addition to CD25, Foxp3 induces high level of miR155 expression to ensure increased IL-2 responsiveness through miR155-mediated down-regulation of the SOCS1 protein (left panel). In the absence of miR155 (right panel), increased amounts of the SOCS1 protein attenuate IL-2R signaling leading to diminished STAT5 phosphorylation and diminished competitive fitness.
Similar articles
- Enhancement of Immunoregulatory Function of Modified Bone Marrow Mesenchymal Stem Cells by Targeting SOCS1.
Zhang X, Hua F, Yang Z, Chen Y, Teng X, Huang H, Zhao Y, Shen Z. Zhang X, et al. Biomed Res Int. 2018 May 8;2018:3530647. doi: 10.1155/2018/3530647. eCollection 2018. Biomed Res Int. 2018. PMID: 29854745 Free PMC article. - Inhibition of SOCS1-/- lethal autoinflammatory disease correlated to enhanced peripheral Foxp3+ regulatory T cell homeostasis.
Collins EL, Jager LD, Dabelic R, Benitez P, Holdstein K, Lau K, Haider MI, Johnson HM, Larkin J 3rd. Collins EL, et al. J Immunol. 2011 Sep 1;187(5):2666-76. doi: 10.4049/jimmunol.1003819. Epub 2011 Jul 25. J Immunol. 2011. PMID: 21788442 Free PMC article. - SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-{gamma} and IL-17A production.
Takahashi R, Nishimoto S, Muto G, Sekiya T, Tamiya T, Kimura A, Morita R, Asakawa M, Chinen T, Yoshimura A. Takahashi R, et al. J Exp Med. 2011 Sep 26;208(10):2055-67. doi: 10.1084/jem.20110428. Epub 2011 Sep 5. J Exp Med. 2011. PMID: 21893603 Free PMC article. - SOCS1 and regulation of regulatory T cells plasticity.
Takahashi R, Yoshimura A. Takahashi R, et al. J Immunol Res. 2014;2014:943149. doi: 10.1155/2014/943149. Epub 2014 Jul 15. J Immunol Res. 2014. PMID: 25133199 Free PMC article. Review. - Regulation of the MIR155 host gene in physiological and pathological processes.
Elton TS, Selemon H, Elton SM, Parinandi NL. Elton TS, et al. Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
- MiR-155 acts as an inhibitory factor in atherosclerosis-associated arterial pathogenesis by down-regulating NoxA1 related signaling pathway in ApoE-/- mouse.
Tang Y, Song H, Shen Y, Yao Y, Yu Y, Wei G, Long B, Yan W. Tang Y, et al. Cardiovasc Diagn Ther. 2021 Feb;11(1):1-13. doi: 10.21037/cdt-20-518. Cardiovasc Diagn Ther. 2021. PMID: 33708473 Free PMC article. - MicroRNAs in myeloproliferative neoplasms.
Zhan H, Cardozo C, Raza A. Zhan H, et al. Br J Haematol. 2013 May;161(4):471-83. doi: 10.1111/bjh.12276. Epub 2013 Feb 25. Br J Haematol. 2013. PMID: 23432162 Free PMC article. Review. - Both MicroRNA-155 and Virus-Encoded MiR-155 Ortholog Regulate TLR3 Expression.
Hu X, Ye J, Qin A, Zou H, Shao H, Qian K. Hu X, et al. PLoS One. 2015 May 4;10(5):e0126012. doi: 10.1371/journal.pone.0126012. eCollection 2015. PLoS One. 2015. PMID: 25938551 Free PMC article. - Noncoding RNAs: the shot callers in tumor immune escape.
Liu L, Wang Q, Qiu Z, Kang Y, Liu J, Ning S, Yin Y, Pang D, Xu S. Liu L, et al. Signal Transduct Target Ther. 2020 Jun 19;5(1):102. doi: 10.1038/s41392-020-0194-y. Signal Transduct Target Ther. 2020. PMID: 32561709 Free PMC article. Review. - IFNγ signaling endows DCs with the capacity to control type I inflammation during parasitic infection through promoting T-bet+ regulatory T cells.
Lee HM, Fleige A, Forman R, Cho S, Khan AA, Lin LL, Nguyen DT, O'Hara-Hall A, Yin Z, Hunter CA, Muller W, Lu LF. Lee HM, et al. PLoS Pathog. 2015 Feb 6;11(2):e1004635. doi: 10.1371/journal.ppat.1004635. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25658840 Free PMC article.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
- Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. - PubMed
- Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–22761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous