Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia - PubMed (original) (raw)
Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia
E Scott Seeley et al. Cancer Res. 2009.
Abstract
Primary cilia have been proposed to participate in the modulation of growth factor signaling pathways. In this study, we determined that ciliogenesis is suppressed in both pancreatic cancer cells and pancreatic intraepithelial neoplasia (PanIN) lesions in human pancreatic ductal adenocarcinoma (PDAC). Primary cilia were absent in these cells even when not actively proliferating. Cilia were also absent from mouse PanIN cells in three different mouse models of PDAC driven by an endogenous oncogenic Kras allele. Inhibition of Kras effector pathways restored ciliogenesis in a mouse pancreatic cancer cell line, raising the possibility that ciliogenesis may be actively repressed by oncogenic Kras. By contrast, normal duct, islet, and centroacinar cells retained primary cilia in both human and mouse pancreata. Thus, arrested ciliogenesis is a cardinal feature of PDAC and its precursor PanIN lesions, does not require ongoing proliferation, and could potentially be targeted pharmacologically.
Figures
Figure 1
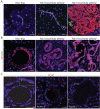
Primary cilia are absent in human PDAC and PanIN lesions. A. PanIN-1. B. PanIN-2. C. Poorly differentiated PDAC. D. PanIN-2/3. A-D: Nuclei (blue). A-C: CK19 (red), acetylated alpha-tubulin (green). D: Ki67 (red), alpha tubulin (green). Insets: A-D, lower left, non-neoplastic ducts from adjacent normal region within respective specimens; and upper right, region of neoplastic ductal epithelium taken at 150% gain and power, revealing a mitotic figure and cytosolic tubules, with absence of ciliary projections. Scale bars: 20μm.
Figure 2
Primary cilia are absent in Kras-dependent murine pancreatic neoplasia. A. Left panel: Centroacinar cells from Pdx1-Cre; middle panel: LSL-KrasG12D (Pdx-Kras), and normal ductule and islet from Pdx1-Cre; right panel: LSL-KrasG12D;Ink4a/Arflox/lox (Pdx-Kras/Ink4a-deleted) murine pancreata. B. Left panel: mPanIN-1 from Pdx-Kras; middle panel: mPanIN-2/3 from Pdx-Kras/Ink4A deleted murine pancreata; right panel: murine pancreatic cancer from same tissue sample as in middle panel. C. Centrosomes, stained with an anti-pericentrin antibody, and primary cilia in normal and neoplastic ducts from Pdx1-Cre;LSL-KrasG12D pancreata. Inset: Magnified view of cilia and centrosomes from cells immediately below the inset. Staining for cytokeratin 19 (CK19), acetylated alpha-tubulin (AT), insulin, and pericentrin (PC), as indicated. Scale bars: 20μm.
Figure 3
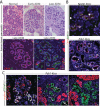
Primary cilia are absent in transitional PanIN lesions. A. Intralobular mPanIN from Pdx1-Cre;LSL-KrasG12D pancreas reveals normal-appearing ductal epithelium (solid white lines), and neoplastic ductal epithelium (dashed lines). Yellow arrowheads indicate transition points between normal and neoplastic epithelium. “Is” indicates islets, CK19 (red), AT (green), and nuclei (blue). Scale bar: 50 μm. B. Magnified region from A, stained as in A, but with anti-Ki67 antibody in white (indicated by white arrowhead). Scale bar: 50 μm. C. Interlobular mPanIN from a Pdx1- Kras pancreas. Staining as in A, scale bar: 20 μm. Inset: Magnified region from C with addition of Ki67 channel in white (indicated by white arrowhead). Scale bar: 50 μm. D. mPanIN from Nestin-Cre;LSL-KrasG12D pancreas stained for CK19 (red), AT (green), Ki67 (white, arrowheads), revealing the terminal cul de sac-like architecture (dashed lines) of a mPanIN lesion appearing to arise from a small ductule (solid lines). Inset: magnified view of ciliated normal ductule from within main image. Scale bar: 20 μm.
Figure 4
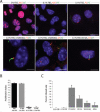
Preservation and reversal of ciliary assembly defect in Rink-1 pancreatic cancer cells prepared from Pdx1-Cre;LSL-KrasG12D;Ink4a/Arflox/lox tumors. A. RInk-1 cells were cultured for 48 hours in the presence of fetal bovine serum (FBS) in the absence or presence of the indicated inhibitors (10 μM each). Cells were then fixed at room temperature and stained for microscopy with antibodies to acetylated alpha tubulin (AT), detyrosinated alpha tubulin (DT), and pericentrin (PC), as indicated. Scale bars: 10 μm. B. Proliferation indices as percentage of cells with nuclear Ki67 and PCNA nuclear positivity following a 48 hour incubation with the indicated FBS concentrations. Data are the means +/− SD from 3 independent experiments. C. Percent ciliated cells following a 48 hour incubation in 0.1% FBS in the absence or presenceof the indicated inhibitors (10 μM each). Data are the means +/− SEM from 3 independent experiments.
Figure 5
Primary cilia are present in ADM lesions. A. Upper images are hematoxylin and eosin stained regions of normal pancreas, and early and late ADM lesions from patients with chronic pancreatitis. Lower images are consecutive serial sections of a late ADM lesion stained with antibodies to cytokeratin 19 (CK19), acetylated alpha tubulin (AT), and amylase, as indicated. Inset: Magnified view of amylase positive cell from within main image. B. Foci of ADM in Nestin-Kras and Pdx-1-Kras pancreata stained with antibodies to CK19, AT, and Ki67, as indicated. C. Proliferation in early ADM (left panel), late ADM (middle panel), and mPanIN (left panel) in Pdx1-Kras pancreata stained with antibodies to amylase, AT, and Ki67 as indicated. Scale bars: 20 μm.
Figure 6
Quantification of primary cilia and proliferation indices in human and murine PanINs, PDAC, and ADM lesions. Data are the means +/− SD from 9 to 12 fields. N: total number of cells scored per grouping.
Similar articles
- Kras mutation rate precisely orchestrates ductal derived pancreatic intraepithelial neoplasia and pancreatic cancer.
Singh K, Pruski M, Bland R, Younes M, Guha S, Thosani N, Maitra A, Cash BD, McAllister F, Logsdon CD, Chang JT, Bailey-Lundberg JM. Singh K, et al. Lab Invest. 2021 Feb;101(2):177-192. doi: 10.1038/s41374-020-00490-5. Epub 2020 Oct 2. Lab Invest. 2021. PMID: 33009500 Free PMC article. - Ciliogenesis and Hedgehog signalling are suppressed downstream of KRAS during acinar-ductal metaplasia in mouse.
Bangs FK, Miller P, O'Neill E. Bangs FK, et al. Dis Model Mech. 2020 Jul 30;13(7):dmm044289. doi: 10.1242/dmm.044289. Dis Model Mech. 2020. PMID: 32571902 Free PMC article. - PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating β-CateninY654.
Gao C, Chen G, Zhang DH, Zhang J, Kuan SF, Hu W, Esni F, Gao X, Guan JL, Chu E, Hu J. Gao C, et al. Cell Mol Gastroenterol Hepatol. 2019;8(4):561-578. doi: 10.1016/j.jcmgh.2019.07.004. Epub 2019 Jul 19. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31330317 Free PMC article. - Critical role of oncogenic KRAS in pancreatic cancer (Review).
Liu J, Ji S, Liang C, Qin Y, Jin K, Liang D, Xu W, Shi S, Zhang B, Liu L, Liu C, Xu J, Ni Q, Yu X. Liu J, et al. Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review. - Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs).
Koorstra JB, Feldmann G, Habbe N, Maitra A. Koorstra JB, et al. Langenbecks Arch Surg. 2008 Jul;393(4):561-70. doi: 10.1007/s00423-008-0282-x. Epub 2008 Feb 19. Langenbecks Arch Surg. 2008. PMID: 18283486 Free PMC article. Review.
Cited by
- Pharmacological inhibition and reversal of pancreatic acinar ductal metaplasia.
da Silva L, Jiang J, Perkins C, Atanasova KR, Bray JK, Bulut G, Azevedo-Pouly A, Campbell-Thompson M, Yang X, Hakimjavadi H, Chamala S, Ratnayake R, Gharaibeh RZ, Li C, Luesch H, Schmittgen TD. da Silva L, et al. Cell Death Discov. 2022 Sep 2;8(1):378. doi: 10.1038/s41420-022-01165-4. Cell Death Discov. 2022. PMID: 36055991 Free PMC article. - Epithelial to Stromal Re-Distribution of Primary Cilia during Pancreatic Carcinogenesis.
Schimmack S, Kneller S, Dadabaeva N, Bergmann F, Taylor A, Hackert T, Werner J, Strobel O. Schimmack S, et al. PLoS One. 2016 Oct 26;11(10):e0164231. doi: 10.1371/journal.pone.0164231. eCollection 2016. PLoS One. 2016. PMID: 27783689 Free PMC article. - Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling.
Hassounah NB, Bunch TA, McDermott KM. Hassounah NB, et al. Clin Cancer Res. 2012 May 1;18(9):2429-35. doi: 10.1158/1078-0432.CCR-11-0755. Epub 2012 Mar 13. Clin Cancer Res. 2012. PMID: 22415315 Free PMC article. - Primary cilium suppression by SREBP1c involves distortion of vesicular trafficking by PLA2G3.
Gijs HL, Willemarck N, Vanderhoydonc F, Khan NA, Dehairs J, Derua R, Waelkens E, Taketomi Y, Murakami M, Agostinis P, Annaert W, Swinnen JV. Gijs HL, et al. Mol Biol Cell. 2015 Jun 15;26(12):2321-32. doi: 10.1091/mbc.E14-10-1472. Epub 2015 Apr 22. Mol Biol Cell. 2015. PMID: 25904332 Free PMC article. - Aurora A and AKT Kinase Signaling Associated with Primary Cilia.
Nishimura Y, Yamakawa D, Shiromizu T, Inagaki M. Nishimura Y, et al. Cells. 2021 Dec 20;10(12):3602. doi: 10.3390/cells10123602. Cells. 2021. PMID: 34944109 Free PMC article. Review.
References
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–9. - PubMed
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. - PubMed
- Schneider L, Clement CA, Teilmann SC, et al. PDGFR alpha signaling is regulated through the primary cilium in fibroblasts. Current Biol. 2005;15:1861–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-127095/CA/NCI NIH HHS/United States
- CA-101306/CA/NCI NIH HHS/United States
- R01 CA102687-05/CA/NCI NIH HHS/United States
- R21 CA127095/CA/NCI NIH HHS/United States
- R01 CA075059/CA/NCI NIH HHS/United States
- R01 CA101306-05/CA/NCI NIH HHS/United States
- R01 CA101306/CA/NCI NIH HHS/United States
- CA-102687/CA/NCI NIH HHS/United States
- R01 CA102687/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous