Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype - PubMed (original) (raw)
Review
Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype
Gillian C Barnett et al. Nat Rev Cancer. 2009 Feb.
Abstract
A key challenge in radiotherapy is to maximize radiation doses to cancer cells while minimizing damage to surrounding healthy tissue. As severe toxicity in a minority of patients limits the doses that can be safely given to the majority, there is interest in developing a test to measure an individual's radiosensitivity before treatment. Variation in sensitivity to radiation is an inherited genetic trait and recent progress in genotyping raises the possibility of genome-wide studies to characterize genetic profiles that predict patient response to radiotherapy.
Figures
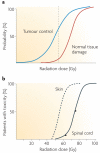
Figure 1. Dose–response curves for radiotherapy
a | The probability of tumour cure increases with increasing radiation dose. As a small volume of normal tissue is unavoidably included in the radiation field, the probability of severe late normal tissue damage also increases. Radiotherapy schedules have developed to maximize cure while minimizing toxicity, and the dotted line shows a theoretical dose associated with ∼60% tumour control and ∼5% severe late toxicity. b | Cumulative frequency dose–response curves. The left-hand curve shows data for skin telangiectasia; the right-hand curve is the putative dose–response curve for spinal cord necrosis. The gradients of the two curves are similar, although the dose at which damage occurs is greater for the spinal cord than skin because of differences in target cells, tissue architecture and cell turnover. Interestingly, inbred animals have an even steeper gradient of the dose–response curve. The principal reason why human clinical data show shallower dose–response curves than inbred animals is inter-individual variability, that is, greater genetic variation, although animal studies are more carefully controlled for factors such as diet, age and co-morbidities than human studies.
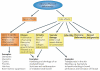
Figure 2. The toxicity of radiotherapy
Acute effects occur during or shortly after completion of treatment and are usually reversible and not generally considered dose-limiting. They occur in rapidly proliferating tissues, such as skin, gastrointestinal tract and the haematopoietic system. Early reactions tend to be relatively insensitive to changes in the radiation dose per fraction but are sensitive to the time over which radiation is delivered. Protracted treatment reduces acute toxicity but can compromise tumour control. Late effects manifest 6 months to several years after radiotherapy. The long time frame prevents titration of radiation dose against toxicity in individual patients, and the relationship between acute and late effects remains unclear,,. As late side effects can be permanent, they provide the basis for dose constraints to radiation toxicity. Late effects typically occur in more slowly proliferating tissues, such as kidney, heart and central nervous system. The pathogenesis includes fibrosis, atrophy and vascular damage. Other important late normal tissue side effects include hormone deficiencies, infertility and second malignancies. Late toxicity tends to be more sensitive to changes in the radiation dose per fraction than acute reacting tissues and less sensitive to the overall treatment time.
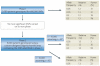
Figure 3. Proposed design for a radiation toxicity genome-wide association study (GWAS)
In the first phase of a staged approach a full set of tagged single nucleotide polymorphisms (tag-SNPs; 600,000) is chosen that comprehensively captures common variations across the genome. This set is genotyped using a whole genome chip in a relatively small population and at a liberal _p_-value threshold, to identify a subset of SNPs with putative associations. As the phenotype of interest exhibits a range of responses, including intermediate levels, this will give greater power than a case–control study of the same size. Phase II takes the top 5% of SNPs identified in phase I or those passing an initial threshold (or filter) and re-tests these SNPs using a custom-designed oligonucleotide array in a larger independent population sample, thus significantly increasing efficiency and reducing genotyping costs.
Similar articles
- Radiation therapy tolerance limits. For one or for all?--Janeway Lecture.
Peters LJ. Peters LJ. Cancer. 1996 Jun 1;77(11):2379-85. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2379::AID-CNCR29>3.0.CO;2-T. Cancer. 1996. PMID: 8635110 Review. - Potential of serum microRNAs as biomarkers of radiation injury and tools for individualization of radiotherapy.
Tomasik B, Chałubińska-Fendler J, Chowdhury D, Fendler W. Tomasik B, et al. Transl Res. 2018 Nov;201:71-83. doi: 10.1016/j.trsl.2018.06.001. Epub 2018 Jun 19. Transl Res. 2018. PMID: 30021695 Review. - Incorporating Genetic Biomarkers into Predictive Models of Normal Tissue Toxicity.
Barnett GC, Kerns SL, Noble DJ, Dunning AM, West CM, Burnet NG. Barnett GC, et al. Clin Oncol (R Coll Radiol). 2015 Oct;27(10):579-87. doi: 10.1016/j.clon.2015.06.013. Epub 2015 Jul 10. Clin Oncol (R Coll Radiol). 2015. PMID: 26166774 Review. - Normal tissue radiosensitivity--how important is it?
Burnet NG, Wurm R, Nyman J, Peacock JH. Burnet NG, et al. Clin Oncol (R Coll Radiol). 1996;8(1):25-34. doi: 10.1016/s0936-6555(05)80035-4. Clin Oncol (R Coll Radiol). 1996. PMID: 8688357 Review. - Prediction of normal tissue response and individualization of doses in radiotherapy.
Guirado D, Ruiz de Almodóvar JM. Guirado D, et al. Phys Med Biol. 2003 Oct 7;48(19):3213-23. doi: 10.1088/0031-9155/48/19/008. Phys Med Biol. 2003. PMID: 14579861
Cited by
- A novel approach to double-strand DNA break analysis through γ-H2AX confocal image quantification and bio-dosimetry.
Valceski M, Engels E, Vogel S, Paino J, Potter D, Hollis C, Khochaiche A, Barnes M, Cameron M, O'Keefe A, Roughley K, Rosenfeld A, Lerch M, Corde S, Tehei M. Valceski M, et al. Sci Rep. 2024 Nov 11;14(1):27591. doi: 10.1038/s41598-024-76683-5. Sci Rep. 2024. PMID: 39528587 Free PMC article. - Association between rheumatoid arthritis and risk of radiotherapy toxicity: a systematic review.
Liebenberg N, McWilliam A, Kerns SL, Marshall DC, West CM. Liebenberg N, et al. BMJ Oncol. 2024 Jan;3(1):e000407. doi: 10.1136/bmjonc-2024-000407. Epub 2024 Jul 16. BMJ Oncol. 2024. PMID: 39524982 - The Influence of the Microbiome on the Complications of Radiotherapy and Its Effectiveness in Patients with Laryngeal Cancer.
Dorobisz K, Dorobisz T, Pazdro-Zastawny K, Czyż K, Janczak M. Dorobisz K, et al. Cancers (Basel). 2024 Nov 1;16(21):3707. doi: 10.3390/cancers16213707. Cancers (Basel). 2024. PMID: 39518144 Free PMC article. - Pilot Feasibility and Safety Study of Hydrogen Gas Inhalation in Locally Advanced Head and Neck Cancer Patients.
Chitapanarux I, Onchan W, Chakrabandhu S, Muangwong P, Autsavapromporn N, Ariyanon T, Akagi J, Mizoo A. Chitapanarux I, et al. Onco Targets Ther. 2024 Oct 29;17:863-870. doi: 10.2147/OTT.S478613. eCollection 2024. Onco Targets Ther. 2024. PMID: 39493677 Free PMC article. Clinical Trial. - Barriers to the Completion of Radiation Therapy in Cervical Cancer Treatment in Nigeria: A Review of Socioeconomic, Geographical, and Psychosocial Factors.
Adebisi AA, Onobun DE, Orji C, Ononye R. Adebisi AA, et al. Cureus. 2024 Oct 3;16(10):e70747. doi: 10.7759/cureus.70747. eCollection 2024 Oct. Cureus. 2024. PMID: 39493197 Free PMC article. Review.
References
- Savitsky K, et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum. Mol. Genet. 1995;4:2025–2032. - PubMed
- Andreassen CN. Can. risk of radiotherapy-induced normal tissue complications be predicted from genetic profiles? Acta Oncol. 2005;44:801–815. - PubMed
- Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin. Radiat. Oncol. 2008;18:126–135. - PubMed
- Easton DF, Eeles RA. Genome-wide association studies in cancer. Hum. Mol. Genet. 2008;17:R109–R115. - PubMed
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Rev. Cancer. 2006;6:702–713. - PubMed
Publication types
MeSH terms
Grants and funding
- 10119/CRUK_/Cancer Research UK/United Kingdom
- 11021/CRUK_/Cancer Research UK/United Kingdom
- 2005NOV40/BCN_/Breast Cancer Now/United Kingdom
- A8740/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials