Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology - PubMed (original) (raw)
Review
Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology
V Krizhanovsky et al. Cold Spring Harb Symp Quant Biol. 2008.
Abstract
Cellular senescence is characterized by an irreversible cell cycle arrest that, when bypassed by mutation, contributes to cellular immortalization. Activated oncogenes induce a hyperproliferative response, which might be one of the senescence cues. We have found that expression of such an oncogene, Akt, causes senescence in primary mouse hepatoblasts in vitro. Additionally, AKT-driven tumors undergo senescence in vivo following p53 reactivation and show signs of differentiation. In another in vivo system, i.e., liver fibrosis, hyperproliferative signaling through AKT might be a driving force of the senescence in activated hepatic stellate cells. Senescent cells up-regulate and secrete molecules that, on the one hand, can reinforce the arrest and, on the other hand, can signal to an innate immune system to clear the senescent cells. The mechanisms governing senescence and immortalization are overlapping with those regulating self-renewal and differentiation. These respective control mechanisms, or their disregulation, are involved in multiple pathological conditions including fibrosis, wound healing, and cancer. Understanding extracellular cues that regulate these processes may enable new therapies for these conditions.
Figures
Figure 1
AKT induces cellular senescence and differentiation in cooperation with p53. (A) Wild-type (WT) but not _p53_−/− primary hepatoblasts senesce in response to AKT. (B) AKT-driven tumor cells senesce in vivo in response to p53 restoration (+DOX), but not in the absence of p53 (−DOX). (C) Expression of the differentiation marker CK8 was increased in tumors following p53 restoration.
Figure 2
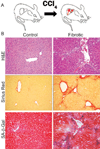
Senescent cells are present in mouse fibrotic liver. (A) Mice were treated with CCl4 twice weekly for 6 weeks. (B) CCl4-treated livers (fibrotic) but not control livers exhibit fibrotic scars (evaluated by H&E and Sirius Red staining). Multiple cells adjacent to the scar stain positively for the senescence marker SA-β-gal.
Figure 3
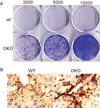
p53−/−;Ink4a/ARF−/− activated stellate cells are immortalized and contribute to fibrosis progression. (A) p53−/−;Ink4a/ARF−/− (DKO) but not wild-type-activated stellate cells form colonies following a 10-day colony-formation assay in vitro as evaluated by crystal violet staining. Numbers indicate amount of cells seeded per well. (B) Immunostaining identified higher expression of the activated stellate cell marker αSMA in fibrotic livers from DKO mice compared to wild-type mice.
Figure 4
AKT signaling might contribute to the senescence of activated stellate cells. pAKT was detected in cells expressing the activated stellate cell marker αSMA in mouse fibrotic livers. Nuclei were identified by DAPI.
Figure 5
The immune system facilitates the clearance of senescent activated stellate cells in vivo. (A) Electron microscopy revealed that immune cells ([lp] lymphocytes; [np] neutrophil) are adjacent to activated HSCs in fibrotic mouse livers but not in normal mouse livers. (B) Mice treated with CCl4 were treated with an anti-NK antibody (to deplete NK cells), polyI:C (as an interferon-γ activator), or saline (as a control) for 10 days. More activated stellate cells, identified by αSMA, are retained in mouse livers following depletion of NK cells.
Figure 6
The eventual senescence of activated stellate cells limits fibrosis through a coordinated program involving cell cycle exit, down-regulation of ECM components, up-regulation of ECM-degrading enzymes, and enhanced immunosurveillance. (Reprinted, with permission, from Krizhanovsky et al. 2008 Supplemental Data [© Elsevier].)
Figure 7
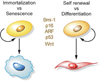
Molecular pathways driving cell fate decisions. Common regulators are illustrated.
Similar articles
- Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives.
Zhang M, Serna-Salas S, Damba T, Borghesan M, Demaria M, Moshage H. Zhang M, et al. Mech Ageing Dev. 2021 Oct;199:111572. doi: 10.1016/j.mad.2021.111572. Epub 2021 Sep 16. Mech Ageing Dev. 2021. PMID: 34536446 Review. - Interleukin-10 induces senescence of activated hepatic stellate cells via STAT3-p53 pathway to attenuate liver fibrosis.
Huang YH, Chen MH, Guo QL, Chen ZX, Chen QD, Wang XZ. Huang YH, et al. Cell Signal. 2020 Feb;66:109445. doi: 10.1016/j.cellsig.2019.109445. Epub 2019 Nov 12. Cell Signal. 2020. PMID: 31730896 - AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy.
Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, Haupt Y, Hannan RD, Pearson RB. Astle MV, et al. Oncogene. 2012 Apr 12;31(15):1949-62. doi: 10.1038/onc.2011.394. Epub 2011 Sep 12. Oncogene. 2012. PMID: 21909130 Free PMC article. - Cellular Senescence: What, Why, and How.
Regulski MJ. Regulski MJ. Wounds. 2017 Jun;29(6):168-174. Wounds. 2017. PMID: 28682291 Review. - Non-cell-autonomous tumor suppression by p53.
Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, Lowe SW. Lujambio A, et al. Cell. 2013 Apr 11;153(2):449-60. doi: 10.1016/j.cell.2013.03.020. Epub 2013 Apr 4. Cell. 2013. PMID: 23562644 Free PMC article.
Cited by
- Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence.
Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, Yen TJ, Zhang R. Tu Z, et al. Dev Cell. 2011 Dec 13;21(6):1077-91. doi: 10.1016/j.devcel.2011.10.010. Epub 2011 Dec 1. Dev Cell. 2011. PMID: 22137763 Free PMC article. - Granule exocytosis mediates immune surveillance of senescent cells.
Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Sagiv A, et al. Oncogene. 2013 Apr 11;32(15):1971-7. doi: 10.1038/onc.2012.206. Epub 2012 Jul 2. Oncogene. 2013. PMID: 22751116 Free PMC article. - Senescent cells and their secretory phenotype as targets for cancer therapy.
Velarde MC, Demaria M, Campisi J. Velarde MC, et al. Interdiscip Top Gerontol. 2013;38:17-27. doi: 10.1159/000343572. Epub 2013 Jan 17. Interdiscip Top Gerontol. 2013. PMID: 23503512 Free PMC article. Review. - Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging.
Vun J, Iqbal N, Jones E, Ganguly P. Vun J, et al. Bioengineering (Basel). 2023 Aug 21;10(8):987. doi: 10.3390/bioengineering10080987. Bioengineering (Basel). 2023. PMID: 37627872 Free PMC article. - Senescent hepatic stellate cells promote liver regeneration through IL-6 and ligands of CXCR2.
Cheng N, Kim KH, Lau LF. Cheng N, et al. JCI Insight. 2022 Jun 16;7(14):e158207. doi: 10.1172/jci.insight.158207. JCI Insight. 2022. PMID: 35708907 Free PMC article.
References
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA008748/CA/NCI NIH HHS/United States
- AG16379/AG/NIA NIH HHS/United States
- R01 AG016379/AG/NIA NIH HHS/United States
- R01 AG016379-09/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous