Fine tuning the cell cycle: activation of the Cdk1 inhibitory phosphorylation pathway during mitotic exit - PubMed (original) (raw)
Fine tuning the cell cycle: activation of the Cdk1 inhibitory phosphorylation pathway during mitotic exit
Tamara A Potapova et al. Mol Biol Cell. 2009 Mar.
Abstract
Inactivation of cyclin-dependent kinase (Cdk) 1 promotes exit from mitosis and establishes G1. Proteolysis of cyclin B is the major known mechanism that turns off Cdk1 during mitotic exit. Here, we show that mitotic exit also activates pathways that catalyze inhibitory phosphorylation of Cdk1, a mechanism previously known to repress Cdk1 only during S and G2 phases of the cell cycle. We present evidence that down-regulation of Cdk1 activates Wee1 and Myt1 kinases and inhibits Cdc25 phosphatase during the M to G1 transition. If cyclin B/Cdk1 complex is present in G1, the inhibitory sites on Cdk1 become phosphorylated. Exit from mitosis induced by chemical Cdk inhibition can be reversed if cyclin B is preserved. However, this reversibility decreases with time after mitotic exit despite the continued presence of the cyclin. We show that this G1 block is due to phosphorylation of Cdk1 on inhibitory residues T14 and Y15. Chemical inhibition of Wee1 and Myt1 or expression of Cdk1 phosphorylation site mutants allows reversal to M phase even from late G1. This late Cdk1 reactivation often results in caspase-dependent cell death. Thus, in G1, the Cdk inhibitory phosphorylation pathway is functional and can lock Cdk1 in the inactive state.
Figures
Figure 1.
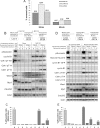
Cells treated for extended periods with the Cdk inhibitor flavopiridol show progressively decreased ability to reactivate Cdk1 and reenter mitosis. (A) Percentages of cells that reenter mitosis after 30 or 60 min of flavopiridol treatment assayed by live videomicroscopy. Before flavopiridol treatment, Xenopus S3 cells were arrested at metaphase by addition of proteasome inhibitor MG132. HeLa cells were arrested with MG132 or by expression of nondegradable R42A cyclinB1-GFP. When used, MG132 was retained in the medium after removal of flavopiridol. Fewer cells reverse after 60-min flavopiridol treatment. (B) Cdk1 kinase activity (phospho-nucleolin); phosphorylation of Cdk1 on T14, Y15, and T161; and protein levels of Wee1, Myt1, Cdc25C, and cyclin B1 were analyzed by Western blotting. Mitotic HeLa cells were collected in nocodazole (lane 0) and induced to exit mitosis by treatment with 5 μM flavopiridol for 30 or 60 min in the absence (lanes 1–4) or presence (lanes 5–8) of proteasome inhibitor MG132. After removal of the Cdk inhibitor, cells were allowed to reenter mitosis for an additional 60 min (lanes 2, 4, 6, and 8). Total Cdk1 protein levels are shown as loading controls. Flavopiridol induces changes in electrophoretic mobility of Wee1/Myt1 kinases and Cdc25C phosphatase that are greater after 60 min of Cdk inhibition. In the presence of proteasome inhibitor that preserves cyclin B, inhibitory phosphorylations on T14 and Y15 are weak at 30 min (lanes 5 and 6) but strong at 60 min (lanes 7 and 8). Cdk1 is robustly reactivated when flavopiridol was washed out after 30 min (lane 6) but only weakly reactivated when flavopiridol was washed out after 60 min (lane 8). (C) Mitotic indices of cell populations induced to exit and reenter mitosis. Mitotic HeLa cells were treated as described in B, fixed, stained with antibody to phospho-histone H3 (mitotic marker) conjugated with Cy5 and processed by flow cytometry. Most cells reenter mitosis when treated with flavopiridol for 30 min before washout (lane 6) but only a minority reenter when treated for 60 min (bar 8). (D) HeLa cells were transfected with wild-type (lanes 0–4) or nondegradable R42A (lanes 5–9) cyclin B-GFP. Mitotic cells were collected in nocodazole and induced to exit mitosis by treatment with 5 μM flavopiridol for 30 or 60 min. After removal of the Cdk inhibitor, cells were allowed to reenter mitosis for an additional 60 min. Cells expressing the wild-type cyclin B1-GFP gradually degrade it upon flavopiridol treatment and do not reenter after flavopiridol washout (lanes 2 and 4). In cells expressing the nondegradable cyclin B, inhibitory phosphorylations on T14 and Y15 are weaker at 30 min (lanes 6 and 7) than at 60 min (lanes 8 and 9). Cdk1 is reactivated more strongly when flavopiridol is washed out after 30 min (lane 7) than 60 min (lane 9). (E) Mitotic indices of cells expressing wild-type or nondegradable cyclin B1 treated as described in D. Cells were fixed, stained with antibody to phospho-histone H3 (mitotic marker) conjugated with Cy5, and processed by flow cytometry. More cells reenter mitosis after 30-min flavopiridol treatment (bar 7) than after 60 min (bar 9).
Figure 2.
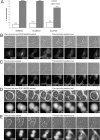
The Wee1 and Myt1 inhibitor PD0166285 allows cells to reenter mitosis from late G1. (A) Percentages of cells that reverse after 60-min flavopiridol treatment in the presence or absence of Wee1/Myt1 inhibitor PD0166285 assayed by live videomicroscopy. Before flavopiridol treatment, Xenopus S3 cells were arrested at metaphase by the addition of the proteasome inhibitor MG132. HeLa cells were arrested with MG132 or by expression of nondegradable R42A cyclin B1-GFP. MG132 and PD0166285 were retained in the medium after removal of flavopiridol. Inhibition of Wee1/Myt1 kinases allows most of the cells to reenter mitosis after 60 min of flavopiridol treatment. (B) Mitotic exit is reversible after 60 min of flavopiridol treatment if both proteasome and Wee1/Myt1 activities are inhibited. An MG132-arrested Xenopus S3 cell was treated with Cdk inhibitor flavopiridol and Wee1/Myt1 inhibitor PD0166285 for 60 min, and then flavopiridol was removed. MG132 and PD016285 were retained after removal of flavopiridol. Supplemental Video 3 shows the complete time-lapse sequence. (C) Mitotic exit is not reversible in most proteasome inhibitor-treated cells after 60 min of flavopiridol treatment if Wee1 and Myt1 are active. An MG132-arrested Xenopus S3 cell was treated with Cdk inhibitor flavopiridol for 60 min, and then flavopiridol was removed. MG132 was retained after removal of flavopiridol. The complete video sequence is shown in Supplemental Video 4. (D) Mitotic exit is reversible after 60 min of flavopiridol treatment if Wee1/Myt1 activities are inhibited in the presence of nondegradable cyclin B1. A HeLa cell expressing R42A cyclin B1-GFP was treated with flavopiridol and Wee1/Myt1 inhibitor PD0166285 for 60 min, and then flavopiridol was removed. PD0166285 was retained after removal of flavopiridol. The complete video sequence is shown in Supplemental Video 5. (E) Mitotic exit is not reversible in most cells expressing nondegradable cyclin B1 after 60 min of flavopiridol if Wee1 and Myt1 are active. A HeLa cell expressing R42A cyclin B1-GFP was treated with flavopiridol for 60 min, and then flavopiridol was removed. The complete video sequence is shown in Supplemental Video 6. The top panels in B-E show phase-contrast images, and the bottom panels show corresponding fluorescent images. Bars, 10 μm.
Figure 3.
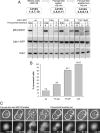
The Wee1 and Myt1 inhibitor PD0166285 prevents inhibitory phosphorylation of Cdk1 during chemically induced mitotic exit and allows Cdk1 to be reactivated in late G1. (A) MG132-treated mitotic HeLa cells (lane 0) were incubated in the Cdk inhibitor flavopiridol without (lane 1) or with (lane 3) the Wee1/Myt1 inhibitor PD 0166285 for 60 min. After removing flavopiridol, cells were allowed to reenter mitosis for an additional 60 min (lanes 2 and 4). Cdk1 kinase activity (phospho-nucleolin), Cdk1 phosphorylation on T14 and Y15, and cyclin B1 protein levels were analyzed by Western blotting. When Wee1 and Myt1 were inhibited by PD0166285, Cdk1 activity was restored after washout of flavopiridol (lane 4). (B) Mitotic HeLa cells expressing nondegradable R42A cyclin B1-GFP (in place of incubation with MG132) were treated and analyzed as described in A. When Wee1 and Myt1 were inhibited by PD0166285, Cdk1 activity was restored after washout of flavopiridol (lane 4). (C) MG132-treated mitotic HeLa cells (lane 0) were incubated in flavopiridol for 60 min (lane 1). Flavopiridol was removed, and cells were incubated for an additional 60 min in medium containing dimethyl sulfoxide (DMSO) (lane 2), Wee1/Myt1 inhibitor PD0166285 (lane 3), or PD0166285 plus the Cdc25 phosphatase inhibitor NSC63284 (lane 4). Samples were analyzed by Western blotting as in described in A. Inhibitory phosphorylations on Cdk1 induced after 60 min of flavopiridol treatment (lane 1) are maintained when flavopiridol is removed (lane 2). Addition of PD0166285 at the time of flavopiridol washout results in dephosphorylation of T14 and Y15 and robust reactivation of Cdk1 (lane 3). Cdk1 dephosphorylation and reactivation was decreased by the addition of the Cdc25 phosphatase inhibitor NSC662385 (lane 4).
Figure 4.
Mutation of inhibitory phosphorylation sites on Cdk1 allows reactivation of Cdk1 kinase activity in late G1. (A) Mitotic HeLa cells expressing wild-type Cdk1-GFP (lanes 1–3), T14A-Cdk1-GFP (lanes 4–6), Y15F-Cdk1-GFP (lanes 7–9), or T14A/Y15F-Cdk1-GFP (lanes 10–12) were blocked in metaphase with MG132. Cells were induced to exit mitosis by treatment with flavopiridol (lanes 2, 5, 8, and 11) for 60 min. Flavopiridol was removed, and cells were allowed to reenter mitosis for an additional 60 min (lanes 3, 6, 9, and 12). Cdk1 kinase activity (phospho-nucleolin), and the protein levels of endogenous Cdk1 and ectopically expressed Cdk1-GFP's were analyzed by Western blotting. Cells expressing mutant Cdk1 proteins show varying degrees of Cdk1 reactivation (lanes 6, 9, and 12) compared with cells expressing wild-type Cdk1.(lane 3). (B) Percentages of HeLa cells expressing wild-type Cdk1-GFP, T14A-Cdk1-GFP, Y15F-Cdk1-GFP, or T14A/Y15F-Cdk1-GFP that underwent reversal from late G1 as assayed by live videomicroscopy. Cells were arrested at metaphase by the addition of the proteasome inhibitor MG132, treated with flavopiridol for 60 min, and then released from flavopiridol. MG132 was retained in the medium. Two cells expressing Y15F-Cdk1-GFP and 4 cells expressing T14A/Y15F-Cdk1-GFP that died during the manipulations were excluded from the analysis. Four cells recondensed chromosomes but maintained an apparently intact nuclear envelope after flavopiridol removal (2 expressing T14A-Cdk1-GFP and 2 expressing Y15F-Cdk1-GFP), and these were scored as reversed. (C) Mitotic exit is reversible after 60 min of flavopiridol treatment when Cdk1 inhibitory phosphorylation sites are ablated. A HeLa cell expressing Y15F-Cdk1-GFP was treated with flavopiridol for 60 min, followed by flavopiridol washout. Cyclin B1 proteolysis was prevented with MG132. The top panel in C shows phase-contrast images, and the bottom panel shows corresponding fluorescent images. Bar, 10 μm. The complete video sequence is shown in Supplemental Video 8.
Figure 5.
Reentry into mitosis from late G1 induces cell death that is dependent on proteasome and caspase activities. (A) Percentages of HeLa cells expressing R42A cyclin B1-GFP that retain viability after reentry into mitosis from late G1 observed by live videomicroscopy. Mitotic cells were treated with flavopiridol and PD0166285 for 60, 90, and 120 min. Flavopiridol was removed but PD0166285 was retained. Cells that failed to reenter mitosis (3 in the 1-h incubation and 1 in the 90-min incubation) were excluded from the analysis of viability. For the control, flavopiridol and PD0166285 were left on for the duration of the experiment. Reentry into mitosis from late G1 induces cell death in cells expressing R42A cyclin B1. (B) A HeLa cell expressing R42A cyclin B1-GFP was treated with flavopiridol and Wee1/Myt1 inhibitor PD0166285 for 120 min. Flavopiridol was removed but PD0166285 retained. Shortly after the flavopiridol washout, cyclin B1-GFP translocates back into the nuclei; the nuclear envelope disassembles, and the chromosomes recondense (arrowheads). Subsequently, the cell undergoes violent blebbing followed by shrinkage and loss of viability. The complete video sequence is shown in Supplemental Video 10. (C) Mitotic HeLa cells expressing R42A cyclin B1-GFP (lane 0) were treated with flavopiridol for 120 min. Flavopiridol was removed for an additional 120 min (lanes 2, 4, 5, and 6) in the presence or absence of PD0166285. Cells in lane 5 were treated with MG132 during the induced mitotic exit and reversal to block proteasome-dependent protein degradation. Cells in lane 6 were treated with the general caspase inhibitor Z-VAD-FMK after flavopiridol removal. Samples were analyzed as described in A; Western blotting of Plk1 was added as an example of a protein that is normally degraded in G1. Histone H3 phosphorylated on serine 10 was used as an additional mitotic marker. Proteasome inhibitor added during mitotic exit and reversal or caspase inhibitor added during reversal allow recovery of Cdk1 activity upon reentry into M phase. (D) The percentage of viable HeLa cells expressing R42A cyclin B1-GFP that retain viability after reentry into mitosis from late G1. Mitotic cells were treated with flavopiridol and PD0166285 for 120 min. Flavopiridol was removed but PD0166285 retained. In the middle column, MG132 was present throughout the experiment to block proteasome dependent protein degradation that normally occurs during mitotic exit and in G1. In the rightmost column, the general caspase inhibitor Z-VAD-FMK was added at the time flavopiridol was removed. Proteasome inhibitor added during mitotic exit and reversal or caspase inhibitor added during reversal rescue cell viability upon reentry into M phase for cells expressing nondegradable cyclin B. (E) The caspase inhibitor Z-VAD-FMK rescues viability during reentry into mitosis from late G1. A HeLa cell expressing R42A cyclin B1-GFP was treated with flavopiridol and PD0166285 for 120 min. Flavopiridol was removed, PD0166285 was retained, and Z-VAD-FMK was added. Shortly after the flavopiridol washout, cyclin B1-GFP translocates back into the nuclei, chromosomes recondense, and cytokinesis reverses. The complete time-lapse sequence is shown in Supplemental Video 12. Top panels in B and E show phase-contrast images, and the bottom panels show corresponding fluorescent images. Bars, 10 μm.
Figure 6.
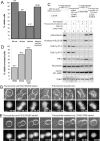
Activation of Wee1 and Myt1 kinases during the M to G1 transition. (A) Asynchronously growing cells, mitotic cells collected by nocodazole shake-off or shake-off cells released from nocodazole block in the presence or absence of 0.5 μM PD0166285 were fixed, stained with propidium iodide, and analyzed by flow cytometry for DNA content. Only single cells were included in the analysis, but the released samples contained increased population of doublets, which were likely telophase cells connected by the midbody. Therefore, G1 peaks in nocodazole released samples may underestimate the actual proportion of cells that have divided. (B) Mitotic HeLa cells were released in the growth medium containing 0.5 μM PD0166285 or DMSO after 2- to 3-h nocodazole block. Cdk1 activity (phospho-nucleolin); phosphorylated histone H3, inhibitory phosphorylations of Cdk1 on T14 and Y15; and protein levels of Wee1, Myt1, Cdc25C, and cyclin B1 were analyzed by Western blotting. Dephosphorylation of nucleolin and histone H3, cyclin B degradation and the electrophoretic mobility shifts of Wee1, Myt1, and Cdc25 were not affected by Wee1 and Myt1 inhibition. However, after nocodazole release antibodies against Cdk1 pT14 and Cdk1pY15 give faint bands, absent in the Wee1/Myt1 inhibitor-treated samples. (C) Lysates for assaying the Myt1 kinase activity were prepared from mitotic HeLa cells released from nocodazole for indicated time or interphase cells. For a substrate, His-tagged kinase-dead (K33R) Cdk1-Δ90cyclin B was used. Substrate was conjugated to magnetic beads and incubated in indicated cell lysates supplemented with ATP. Beads were then pulled out, washed, and the bound His-Cdk1 was analyzed for inhibitory phosphorylation on T14 by Western blotting. The substrate gradually became phosphorylated on the T14 as cells exited mitosis. By 4 h after nocodazole release levels of T14 phosphorylation were comparable with that of interphase cells. The absence of ATP or addition of 5 μM Wee1/Myt1 inhibitor PD0166285 prevented the T14 phosphorylation of the substrate. Lane labeled “mock” contained empty beads (without the substrate) incubated with interphase cell lysates and ATP, lane labeled “beads” contained substrate-conjugated beads not incubated in any lysate. To control for loading, the same PVDF membrane was stained with Coomassie Blue. The kinase-containing lysates were analyzed for the Myt1 electrophoretic mobility shift by Western blotting. The shift to the faster mobility, active form corresponded to the phosphorylation of the substrate on T14.
Similar articles
- Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited.
Potapova TA, Sivakumar S, Flynn JN, Li R, Gorbsky GJ. Potapova TA, et al. Mol Biol Cell. 2011 Apr 15;22(8):1191-206. doi: 10.1091/mbc.E10-07-0599. Epub 2011 Feb 16. Mol Biol Cell. 2011. PMID: 21325631 Free PMC article. - A two-step inactivation mechanism of Myt1 ensures CDK1/cyclin B activation and meiosis I entry.
Ruiz EJ, Vilar M, Nebreda AR. Ruiz EJ, et al. Curr Biol. 2010 Apr 27;20(8):717-23. doi: 10.1016/j.cub.2010.02.050. Epub 2010 Apr 1. Curr Biol. 2010. PMID: 20362450 - Role for non-proteolytic control of M-phase-promoting factor activity at M-phase exit.
D'Angiolella V, Palazzo L, Santarpia C, Costanzo V, Grieco D. D'Angiolella V, et al. PLoS One. 2007 Feb 28;2(2):e247. doi: 10.1371/journal.pone.0000247. PLoS One. 2007. PMID: 17327911 Free PMC article. - PP2A function toward mitotic kinases and substrates during the cell cycle.
Jeong AL, Yang Y. Jeong AL, et al. BMB Rep. 2013 Jun;46(6):289-94. doi: 10.5483/bmbrep.2013.46.6.041. BMB Rep. 2013. PMID: 23790971 Free PMC article. Review. - Regulation of Cdc2 activity by phosphorylation at T14/Y15.
Berry LD, Gould KL. Berry LD, et al. Prog Cell Cycle Res. 1996;2:99-105. doi: 10.1007/978-1-4615-5873-6_10. Prog Cell Cycle Res. 1996. PMID: 9552387 Review.
Cited by
- Transmembrane signal transduction in oocyte maturation and fertilization: focusing on Xenopus laevis as a model animal.
Sato K. Sato K. Int J Mol Sci. 2014 Dec 23;16(1):114-34. doi: 10.3390/ijms16010114. Int J Mol Sci. 2014. PMID: 25546390 Free PMC article. Review. - Stochastic effects as a force to increase the complexity of signaling networks.
Kuwahara H, Gao X. Kuwahara H, et al. Sci Rep. 2013;3:2297. doi: 10.1038/srep02297. Sci Rep. 2013. PMID: 23892365 Free PMC article. - Regulated inactivation of the spindle assembly checkpoint without functional mitotic spindles.
De Souza CP, Hashmi SB, Yang X, Osmani SA. De Souza CP, et al. EMBO J. 2011 Jun 3;30(13):2648-61. doi: 10.1038/emboj.2011.176. EMBO J. 2011. PMID: 21642954 Free PMC article. - Coupling of T161 and T14 phosphorylations protects cyclin B-CDK1 from premature activation.
Coulonval K, Kooken H, Roger PP. Coulonval K, et al. Mol Biol Cell. 2011 Nov;22(21):3971-85. doi: 10.1091/mbc.E11-02-0136. Epub 2011 Sep 7. Mol Biol Cell. 2011. PMID: 21900495 Free PMC article. - A haploid genetic screen identifies the G1/S regulatory machinery as a determinant of Wee1 inhibitor sensitivity.
Heijink AM, Blomen VA, Bisteau X, Degener F, Matsushita FY, Kaldis P, Foijer F, van Vugt MA. Heijink AM, et al. Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15160-5. doi: 10.1073/pnas.1505283112. Epub 2015 Nov 23. Proc Natl Acad Sci U S A. 2015. PMID: 26598692 Free PMC article.
References
- Allan L. A., Clarke P. R. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol. Cell. 2007;26:301–310. - PubMed
- Becker E. B., Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 2004;72:1–25. - PubMed
- Boutros R., Dozier C., Ducommun B. The when and wheres of CDC25 phosphatases. Curr. Opin. Cell Biol. 2006;18:185–191. - PubMed
- Cheng A., Xiong W., Ferrell J. E., Jr., Solomon M. J. Identification and comparative analysis of multiple mammalian Speedy/Ringo proteins. Cell Cycle. 2005;4:155–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous