AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA - PubMed (original) (raw)
. 2009 Mar 26;458(7237):509-13.
doi: 10.1038/nature07710. Epub 2009 Jan 21.
Affiliations
- PMID: 19158676
- PMCID: PMC2862225
- DOI: 10.1038/nature07710
AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA
Teresa Fernandes-Alnemri et al. Nature. 2009.
Abstract
Host- and pathogen-associated cytoplasmic double-stranded DNA triggers the activation of a NALP3 (also known as cryopyrin and NLRP3)-independent inflammasome, which activates caspase-1 leading to maturation of pro-interleukin-1beta and inflammation. The nature of the cytoplasmic-DNA-sensing inflammasome is currently unknown. Here we show that AIM2 (absent in melanoma 2), an interferon-inducible HIN-200 family member that contains an amino-terminal pyrin domain and a carboxy-terminal oligonucleotide/oligosaccharide-binding domain, senses cytoplasmic DNA by means of its oligonucleotide/oligosaccharide-binding domain and interacts with ASC (apoptosis-associated speck-like protein containing a CARD) through its pyrin domain to activate caspase-1. The interaction of AIM2 with ASC also leads to the formation of the ASC pyroptosome, which induces pyroptotic cell death in cells containing caspase-1. Knockdown of AIM2 by short interfering RNA reduced inflammasome/pyroptosome activation by cytoplasmic DNA in human and mouse macrophages, whereas stable expression of AIM2 in the non-responsive human embryonic kidney 293T cell line conferred responsiveness to cytoplasmic DNA. Our results show that cytoplasmic DNA triggers formation of the AIM2 inflammasome by inducing AIM2 oligomerization. This study identifies AIM2 as an important inflammasome component that senses potentially dangerous cytoplasmic DNA, leading to activation of the ASC pyroptosome and caspase-1.
Figures
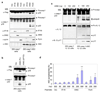
Figure 1. Activation of caspase-1 by AIM2
a, b, The indicated cells in (1 × 106 cells/35 mm well) were transfected with 0.5 µg of an empty vector (vec) or constructs encoding human AIM2, IFI16 or pyrin (a), or vec, AIM2 or AIM2-ΔPYD plasmids (b) as indicated for 24 h. Lysates were analyzed by western blotting with anti-Flag antibody (1st panel from top), or anti-caspase-1 p30 polyclonal antibody (2nd panel from top). The blot in (a) was re-probed with IFI16, AIM2, pyrin or ASC antibodies (3rd-6th panel from top, respectively). c, Immunobloting for caspase-1 (upper panel), IL-1β (middle panel) and IL-1β p17 (lower panel) in culture supernatants of the indicated cell lines transfected with the indicated amounts of AIM2 expression plasmid. d, Percentages of ASC pyroptosomes in 293-ASC-EGFP-N1 cells following transfection with the indicated plasmids. Values represent mean ± S.D. (n = 4); *, P<0.05; **, P<0.01;***, P<0.005. casp1 or casp-1 refer to caspase-1; Procasp1 refers to Pro-caspase-1; 293 refers to 293T; α- refers to Anti-
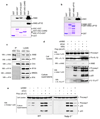
Figure 2. AIM2 interacts with ASC to activate caspase-1
a, b, Immunoblots showing in vitro association of ASC-GST and ASC-PYD-GST with full-length AIM2 (upper panel), but not with truncated AIM2-ΔPYD (middle panel) (a), and in vitro association of AIM2-GST with full-length ASC (b). The lower panels show Coomassie stained gel of the GST-tagged proteins used in the pull-down assay (see Supplementary Fig. 3). GST–ASC-CARD refers to the isolated CARD of ASC fused to GST. c, Association of endogenous ASC with endogenous AIM2 in THP-1 cell lysate detected by immunoprecipitation (IP, 1st lanes, left panels) with an ASC-specific monoclonal antibody as described in the “Methods”. IP with an Omi-specific monoclonal antibody was used as a negative control (2nd lanes, left panels). d, e, Immunoblots of lysates and culture supernatants of THP-1 cells (d) or mouse WT and NALP3−/− bone marrow macrophages (e) transfected with AIM2-targeting siRNA (siAIM2) or control non-specific siRNA (siCon) followed by transfection with poly (dA:dT) or treatment with MSU as indicated. The release of the active p20 subunit and some procaspase-1 in the culture supernatants is a result of cell death and lysis (see Fig. 6, and Supplementary movie 1).
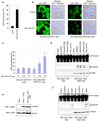
Figure 3. Cell-based reconstitution of the AIM2 inflammasome
a, Percentages of ASC pyroptosomes in untransfected (none) or poly (dA:dT)-transfected THP-1 cells. Values represent mean and s.d. (n = 3). b, Fluorescence confocal images showing formation of the speck-like ASC pyroptosomes only in 293-AIM2-ASC-EGFP-N1 cells (right panels), but not in 293-ASC-EGFP-N1 cells (left panels), 2 h after transfection with vehicle (control, upper panels) or poly (dA:dT) (lower panels). DIC, Differential Interference Contrast. c, Percentages of ASC pyroptosomes in the indicated cell lines following transfection with poly (dA:dT) for 2h. Values represent mean ± S.D. (n = 7); *, P<0.005; **, P<0.001. d, Immunoblot for caspase-1 in lysates from 293-caspase-1-ASC (1st to 5th lanes) or 293-caspase-1-ASC-AIM2 (6th to 10th lanes) cells following transfection with the indicated types of DNA (0.5 µg/1 × 106 cells) for 24 h. e, Immunoblot for AIM2 (upper panel) or Omi (lower panel) in lysates from uninduced (1st lane) or interferon γ-induced (2nd lane) THP-1 cells, or 293-caspase-1-ASC-AIM2 cells (3rd lane). f, Immunoblot for caspase-1 in lysates from 293-caspase-1-ASC (1st to 4th lanes) or 293-caspase-1-ASC-NALP3 (5th to 8th lanes) cells following transfection with the indicated plasmid DNA (0.5 µg /1 × 106 cells) for 24 h.
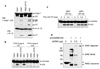
Figure 4. In vitro reconstitution of the AIM2 inflammasome
a, Immunoblot for caspase-1 (upper panel) or IL-1β (lower panel) following in vitro incubation of PYD-caspase-1 and pro-IL-1β with purified GST-AIM2 (2nd lane), GST-AIM2-ΔPYD (3rd lane) or ASC pyroptosome (4th lane). b, c, Autoradiograms of 35S-labeled WT or C287A chimeric PYD-caspase-9 following in vitro incubation with purified GST-AIM2 (2nd and 6th lanes), GST-AIM2-ΔPYD (3rd and 7th lanes) or ASC pyroptosome (4th and 8th lanes) (b) or with the indicated amounts of purified GST-AIM2 in the presence or absence of the indicated amounts of poly (dA:dT) (c). d, Immunoblot for AIM2 after cross-linking of purified AIM2 (2 µg) with glutaraldehyde in the presence of the indicated amounts of 64-mer dsDNA. A longer exposure of this gel is shown in Supplementary Fig. 8.
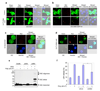
Figure 5. Cytoplasmic DNA-induced AIM2 oligomerization and pyroptosis
a, b, Confocal live cell images of 293-EGFP cells (a) or 293-AIM2-EGFP-N1 (b) following transfection with vehicle (control, upper panels) or Cy™3-labeled DNA (red, lower panels). c, d, Confocal live cell images of NALP3−/− -AIM2-EGFP-N1 bone marrow macrophages following transfection with vehicle (c) or Cy™3-labeled DNA (d). Notice the oligomerization of AIM2-GFP by the red DNA in the 293-AIM2-EGFP-N1 cells (b, bottom panels) and NALP3−/− -AIM2-EGFP-N1 macrophages (d). The green (AIM2-EGFP), red (Cy™3-labeled DNA), gray (DIC), and blue (Hoechst stain, nucleus) channels, and the merged channels are indicated. The two white arrows (lower-second panel from left in (d)) indicate staining of the cytoplasmic DNA with the blue Hoechst stain, which specifically stains DNA. Notice the pyroptotic cell death features induced by the Cy™3-labeled DNA in NALP3−/− -AIM2-EGFP-N1 macrophages (d), but not in the 293-AIM2-EGFP-N1 (b). e, Immunoblot for ASC showing the presence of the oligomeric ASC pyroptosomes only in the pellets of poly (dA:dT)-transfected (6th lane), but not in the pellets of untransfected (5th lane), NALP3−/− macrophages. A shorter exposure and a more detailed legend of this gel is shown in Supplementary Fig. 12. f, Percentages of LDH release into the culture medium of NALP3−/− macrophages transfected with vehicle (1st and 2nd columns), non-specific control siRNA (siCon, 3rd and 4th columns) or mouse AIM2-specific siRNA (siAIM2, 5th and 6th columns) followed by transfection with or without poly (dA:dT) (1 µg/1 × 106 cells) as indicated. Values represent mean ± S.D. (n = 3); *, P < 0.001.
Similar articles
- AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC.
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. Hornung V, et al. Nature. 2009 Mar 26;458(7237):514-8. doi: 10.1038/nature07725. Epub 2009 Jan 21. Nature. 2009. PMID: 19158675 Free PMC article. - AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC.
Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ. Sagulenko V, et al. Cell Death Differ. 2013 Sep;20(9):1149-60. doi: 10.1038/cdd.2013.37. Epub 2013 May 3. Cell Death Differ. 2013. PMID: 23645208 Free PMC article. - NLRC3 protein inhibits inflammation by disrupting NALP3 inflammasome assembly via competition with the adaptor protein ASC for pro-caspase-1 binding.
Eren E, Berber M, Özören N. Eren E, et al. J Biol Chem. 2017 Jul 28;292(30):12691-12701. doi: 10.1074/jbc.M116.769695. Epub 2017 Jun 5. J Biol Chem. 2017. PMID: 28584053 Free PMC article. - AIM2 Inflammasome Assembly and Signaling.
Wang B, Tian Y, Yin Q. Wang B, et al. Adv Exp Med Biol. 2019;1172:143-155. doi: 10.1007/978-981-13-9367-9_7. Adv Exp Med Biol. 2019. PMID: 31628655 Review. - Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.
de Lima JD, de Paula AGP, Yuasa BS, de Souza Smanioto CC, da Cruz Silva MC, Dos Santos PI, Prado KB, Winter Boldt AB, Braga TT. de Lima JD, et al. Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
- Biting the hand that feeds: Metabolic determinants of cell fate during infection.
Fraschilla I, Evavold CL. Fraschilla I, et al. Front Immunol. 2022 Oct 13;13:923024. doi: 10.3389/fimmu.2022.923024. eCollection 2022. Front Immunol. 2022. PMID: 36311735 Free PMC article. Review. - Clinical features and genetic background of the periodic Fever syndrome with aphthous stomatitis, pharyngitis, and adenitis: a single center longitudinal study of 81 patients.
Perko D, Debeljak M, Toplak N, Avčin T. Perko D, et al. Mediators Inflamm. 2015;2015:293417. doi: 10.1155/2015/293417. Epub 2015 Mar 4. Mediators Inflamm. 2015. PMID: 25821352 Free PMC article. - Neutrophil sensing of cytoplasmic, pathogenic DNA in a cGAS-STING-independent manner.
Yu Z, Chen T, Cao X. Yu Z, et al. Cell Mol Immunol. 2016 Jul;13(4):411-4. doi: 10.1038/cmi.2015.34. Epub 2015 Apr 27. Cell Mol Immunol. 2016. PMID: 25914935 Free PMC article. No abstract available. - AIM2 Inhibits BRAF-Mutant Colorectal Cancer Growth in a Caspase-1-Dependent Manner.
Shah S, Qin S, Luo Y, Huang Y, Jing R, Shah JN, Chen J, Chen H, Zhong M. Shah S, et al. Front Cell Dev Biol. 2021 Mar 25;9:588278. doi: 10.3389/fcell.2021.588278. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842454 Free PMC article. - Caspase functions in cell death and disease.
McIlwain DR, Berger T, Mak TW. McIlwain DR, et al. Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a008656. doi: 10.1101/cshperspect.a008656. Cold Spring Harb Perspect Biol. 2013. PMID: 23545416 Free PMC article. Review.
References
- Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. - PubMed
- Ludlow LE, Johnstone RW, Clarke CJ. The HIN-200 family: more than interferon-inducible genes? Exp Cell Res. 2005;308:1–17. - PubMed
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous