Recent advances of novel targeted therapy in non-small cell lung cancer - PubMed (original) (raw)
Review
Recent advances of novel targeted therapy in non-small cell lung cancer
Jed A Katzel et al. J Hematol Oncol. 2009.
Abstract
Lung cancer is the leading cause of cancer deaths world-wide. Recent advances in cancer biology have led to the identification of new targets in neoplastic cells and the development of novel targeted therapies. At this time, two targeted agents are approved by the FDA in advanced non-small cell lung cancer (NSCLC): the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib, and the anitangiogenic bevacizumab. A third agent, cetuximab, which was recently shown to enhance survival when used with cisplatin and vinorelbine as first line therapy for advanced NSCLC, will likely be approved by regulatory agencies. With more than 500 molecularly targeted agents under development, the prospects of identifying novel therapies that benefit individual patients with lung cancer are bright.
Figures
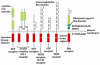
Figure 1
Tyrosine Kinase Receptor (RTK) families. Adapted by permission from Macmillan Publishers Ltd: The Biology of Cancer, Garland Science, 2007.
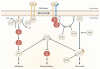
Figure 2
EGFR signaling pathways. Two important cell-survival pathways that operate downstream of activated ErbB transmembrane receptor tyrosine kinases (represented by pairs of yellow, and yellow and blue receptors to represent homo- and hetero-dimers, respectively), along with some of the key constituent signaling molecules are shown. The Ras-Raf-MEK-ERK pathway is shown on the left, and the phosphatidylinositol 3-kinase (PI3K)-AKT pathway is shown on the right. Key points along the pathway where targeted inhibition seems to exert a blockade are indicated by red circles, showing the relevant proteins they target. ERK, extracellular signal-regulated kinase; GRB2, growth factor receptor-bound protein 2; mTOR, mammalian target of rapamycin; SOS, son of sevenless. Used with permission from: Nature Reviews 2007 Sharma et al. Pg 177.
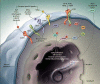
Figure 3
EGFR signal transduction pathways. Three steps can be schematically defined in the activation of EGFR-dependent intracellular signaling. First, the binding of a receptor-specific ligand occurs in the extracellular portion of the EGFR or of one of the EGFR-related receptors (HER2, HER3, or HER4). Second, the formation of a functionally active EGFR-EGFR dimer (homodimer) or an EGFR-HER2, EGFR-HER3, or EGFR-HER4 dimer (heterodimer) causes the ATP-dependent phosphorylation of specific tyrosine residues in the EGFR intracellular domain. Third, this phosphorylation triggers a complex program of intracellular signals to the cytoplasm and then to the nucleus. The two major intracellular pathways activated by EGFR are the RAS-RAF-MEK-MAPK pathway, which controls gene transcription, cell-cycle progression from the G1 phase to the S phase, and cell proliferation, and the PI3K-Akt pathway, which activates a cascade of anti-apoptotic and prosurvival signals. bFGF, basic fibroblast growth factor, HB-EGF, heparin-binding EGF, MAPK, mitogen-activated protein kinase, PI3K, phosphatidylinositol 3,4,5-kinase, TGFa transforming growth factor alpha, and VEGF, vascular endothelial growth factor. Used with permission from: NEJM 2008 Ciardiello et al.).
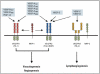
Figure 4
VEGF signaling pathways. Binding specificity of various vascular endothelial growth factor (VEGF) family members and their receptors. The VEGF family consists of seven ligands derived from distinct genes (VEGF-A, -B, -C, -D, and -E, placenta growth factor [PIGF] -1 and -2). VEGF family members have specific binding affinities to VEGF receptor (VEGFR) -1, VEGFR-2 and BEGFR-3 tyrosine kinase receptors as shown. In addition, neuropilin (NRP)-1 and NRP-2 are co-receptors for specific isoforms of VEGF family members and increase binding affinity of these ligands to their respective receptors. Used with permission from: Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005; 23: 1011–27.
Similar articles
- First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.
Greenhalgh J, Boland A, Bates V, Vecchio F, Dundar Y, Chaplin M, Green JA. Greenhalgh J, et al. Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD010383. doi: 10.1002/14651858.CD010383.pub3. Cochrane Database Syst Rev. 2021. PMID: 33734432 Free PMC article. - Advances in molecular-based personalized non-small-cell lung cancer therapy: targeting epidermal growth factor receptor and mechanisms of resistance.
Jotte RM, Spigel DR. Jotte RM, et al. Cancer Med. 2015 Nov;4(11):1621-32. doi: 10.1002/cam4.506. Epub 2015 Aug 26. Cancer Med. 2015. PMID: 26310719 Free PMC article. Review. - Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer.
Gao J, Li HR, Jin C, Jiang JH, Ding JY. Gao J, et al. Clin Transl Oncol. 2019 Oct;21(10):1287-1301. doi: 10.1007/s12094-019-02075-1. Epub 2019 Mar 12. Clin Transl Oncol. 2019. PMID: 30864018 Review. - First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.
Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. Greenhalgh J, et al. Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. PMID: 27223332 Updated. Review. - Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors.
Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Westover D, et al. Ann Oncol. 2018 Jan 1;29(suppl_1):i10-i19. doi: 10.1093/annonc/mdx703. Ann Oncol. 2018. PMID: 29462254 Free PMC article. Review.
Cited by
- Clinical relevance of KRAS in human cancers.
Jancík S, Drábek J, Radzioch D, Hajdúch M. Jancík S, et al. J Biomed Biotechnol. 2010;2010:150960. doi: 10.1155/2010/150960. Epub 2010 Jun 7. J Biomed Biotechnol. 2010. PMID: 20617134 Free PMC article. Review. - Erlotinib resistance is altered after gemcitabine chemotherapy for recurrent non-small-cell lung cancer.
Wang Y, Zhang J, Liu H, Yuan S, Wang F, Ning K, Liu F, Yu J. Wang Y, et al. Clin Drug Investig. 2011;31(4):279-83. doi: 10.2165/00044011-200930100-8. Clin Drug Investig. 2011. PMID: 21080748 - Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA.
Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Taratula O, et al. J Control Release. 2013 Nov 10;171(3):349-57. doi: 10.1016/j.jconrel.2013.04.018. Epub 2013 May 3. J Control Release. 2013. PMID: 23648833 Free PMC article. - Immunotherapy in the landscape of new targeted treatments for non-small cell lung cancer.
Gérard C, Debruyne C. Gérard C, et al. Mol Oncol. 2009 Dec;3(5-6):409-24. doi: 10.1016/j.molonc.2009.09.001. Epub 2009 Sep 10. Mol Oncol. 2009. PMID: 19846354 Free PMC article. Review. - Emodin reverses 5-Fu resistance in human colorectal cancer via downregulation of PI3K/Akt signaling pathway.
Li T, Si W, Zhu J, Yin L, Zhong C. Li T, et al. Am J Transl Res. 2020 May 15;12(5):1851-1861. eCollection 2020. Am J Transl Res. 2020. PMID: 32509181 Free PMC article.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Ca Cancer J Clin. 2005;55:74–108. - PubMed
- Weinberg RA. Growth factor receptors can function as oncoproteins. In: Elizabeth Zayatz, editor. The biology of cancer. 1. New York: Garland Science; 2007. pp. 129–131.
- Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous