The prion protein knockout mouse: a phenotype under challenge - PubMed (original) (raw)
The prion protein knockout mouse: a phenotype under challenge
Andrew D Steele et al. Prion. 2007 Apr-Jun.
Abstract
The key pathogenic event in prion disease involves misfolding and aggregation of the cellular prion protein (PrP). Beyond this fundamental observation, the mechanism by which PrP misfolding in neurons leads to injury and death remains enigmatic. Prion toxicity may come about by perverting the normal function of PrP. If so, understanding the normal function of PrP may help to elucidate the molecular mechansim of prion disease. Ablation of the Prnp gene, which encodes PrP, was instrumental for determining that the continuous production of PrP is essential for replicating prion infectivity. Since the structure of PrP has not provided any hints to its possible function, and there is no obvious phenotype in PrP KO mice, studies of PrP function have often relied on intuition and serendipity. Here, we enumerate the multitude of phenotypes described in PrP deficient mice, many of which manifest themselves only upon physiological challenge. We discuss the pleiotropic phenotypes of PrP deficient mice in relation to the possible normal function of PrP. The critical question remains open: which of these phenotypes are primary effects of PrP deletion and what do they tell us about the function of PrP?
Figures
Figure 1
The overlap between the normal function of PrPC and the pathogenic dysfunction of PrPSc in disease depicted as a Venn diagram. The extent of overlap between the normal function of PrPC and its role in prion disease is open to speculation, the circles could have a much greater or perhaps even less overlap.
Figure 2
Different approaches to study PrPC's normal function. There are many ways to approach the study of the normal function of PrPC, none of which have conclusively demonstrated PrPC's function. Interacting partners of PrPC have yielded many interesting candidates, human genetic studies have found associations of PrPC with diseases beyond prion disease and even to learning and memory, over- and ectopic-expression studies constitute another approach to determine the function of PrPC, and finally, the focus of this review, the PrP KO has given some clues to the function of PrPC.
Figure 3
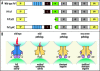
A model for the effects of PrPC deletion and deletion mutants of PrPC. (A) Schematic diagram of wild-type PrPC and deletion mutants. SP, signal peptide; octapeptide repeats are indicated in blue; CC, charge cluster; HC, hydrophobic core; H1, H2, H3 Helix 1,2 and 3, respectively; GFP, GPI-anchor addition sequence (B). PrP (black) consists of a globular C-terminal domain (hexagon) and a N-terminal flexible tail (arch) encompassing the octapeptide repeats (ORs) (circle). The model rests on the following assumptions: (1) PrP activates a hitherto unidentified receptor (PrPR) which transmits myelin maintenance signals (flashes); (2) in the absence of PrP, PrPR exerts some residual activity, either constitutively or by recruiting a surrogate ligand; (3) the activity of PrP and its mutants requires homo- or heterodimerization, and induces dimerization of PrPR; and (4) PrP dimers containing PrPΔCD or PrPΔCD trap PrPR in an inactive dominant-negative state. Finally, (5) the OR region stabilizes the interaction between PrP and PrPR, but does not contribute directly to signaling.
Similar articles
- Prion neurotoxicity: insights from prion protein mutants.
Solomon IH, Schepker JA, Harris DA. Solomon IH, et al. Curr Issues Mol Biol. 2010;12(2):51-61. Epub 2009 Sep 18. Curr Issues Mol Biol. 2010. PMID: 19767650 Free PMC article. Review. - Requirements for mutant and wild-type prion protein misfolding in vitro.
Noble GP, Walsh DJ, Miller MB, Jackson WS, Supattapone S. Noble GP, et al. Biochemistry. 2015 Feb 10;54(5):1180-7. doi: 10.1021/bi501495j. Epub 2015 Jan 22. Biochemistry. 2015. PMID: 25584902 Free PMC article. - Small is not beautiful: antagonizing functions for the prion protein PrP(C) and its homologue Dpl.
Behrens A, Aguzzi A. Behrens A, et al. Trends Neurosci. 2002 Mar;25(3):150-4. doi: 10.1016/s0166-2236(00)02089-0. Trends Neurosci. 2002. PMID: 11852147 Review. - Prion protein with an octapeptide insertion has impaired neuroprotective activity in transgenic mice.
Li A, Piccardo P, Barmada SJ, Ghetti B, Harris DA. Li A, et al. EMBO J. 2007 Jun 6;26(11):2777-85. doi: 10.1038/sj.emboj.7601726. Epub 2007 May 17. EMBO J. 2007. PMID: 17510630 Free PMC article. - The N-Terminal Polybasic Region of Prion Protein Is Crucial in Prion Pathogenesis Independently of the Octapeptide Repeat Region.
Das NR, Miyata H, Hara H, Chida J, Uchiyama K, Masujin K, Watanabe H, Kondoh G, Sakaguchi S. Das NR, et al. Mol Neurobiol. 2020 Feb;57(2):1203-1216. doi: 10.1007/s12035-019-01804-5. Epub 2019 Nov 9. Mol Neurobiol. 2020. PMID: 31707632
Cited by
- Impairment of cerebellar long-term depression and GABAergic transmission in prion protein deficient mice ectopically expressing PrPLP/Dpl.
Kishimoto Y, Hirono M, Atarashi R, Sakaguchi S, Yoshioka T, Katamine S, Kirino Y. Kishimoto Y, et al. Sci Rep. 2020 Sep 28;10(1):15900. doi: 10.1038/s41598-020-72753-6. Sci Rep. 2020. PMID: 32985542 Free PMC article. - Reduced Hyperpolarization-Activated Current Contributes to Enhanced Intrinsic Excitability in Cultured Hippocampal Neurons from PrP(-/-) Mice.
Fan J, Stemkowski PL, Gandini MA, Black SA, Zhang Z, Souza IA, Chen L, Zamponi GW. Fan J, et al. Front Cell Neurosci. 2016 Mar 24;10:74. doi: 10.3389/fncel.2016.00074. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27047338 Free PMC article. - The elusive role of the prion protein and the mechanism of toxicity in prion disease.
Chiesa R. Chiesa R. PLoS Pathog. 2015 May 7;11(5):e1004745. doi: 10.1371/journal.ppat.1004745. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25951168 Free PMC article. Review. No abstract available. - Transcriptomic analysis of zebrafish prion protein mutants supports conserved cross-species function of the cellular prion protein.
Pollock NM, Leighton P, Neil G, Allison WT. Pollock NM, et al. Prion. 2021 Dec;15(1):70-81. doi: 10.1080/19336896.2021.1924557. Prion. 2021. PMID: 34139950 Free PMC article. - Prion-like disorders: blurring the divide between transmissibility and infectivity.
Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Cushman M, et al. J Cell Sci. 2010 Apr 15;123(Pt 8):1191-201. doi: 10.1242/jcs.051672. J Cell Sci. 2010. PMID: 20356930 Free PMC article.
References
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: Current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. - PubMed
- Hetz C, Maundrell K, Soto C. Is loss of function of the prion protein the cause of prion disorders? Trends Mol Med. 2003;9:237–243. - PubMed
- Aguzzi A. Prion diseases of humans and farm animals: Epidemiology, genetics, and pathogenesis. J Neurochem. 2006;97:1726–1739. - PubMed
- Caughey B, Baron GS. Prions and their partners in crime. Nature. 2006;443:803–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials