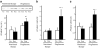Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial - PubMed (original) (raw)
Randomized Controlled Trial
Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial
D K Coletta et al. Diabetologia. 2009 Apr.
Abstract
Aims/hypothesis: The molecular mechanisms by which thiazolidinediones improve insulin sensitivity in type 2 diabetes are not fully understood. We hypothesised that pioglitazone would activate the adenosine 5'-monophosphate-activated protein kinase (AMPK) pathway and increase the expression of genes involved in adiponectin signalling, NEFA oxidation and mitochondrial function in human skeletal muscle.
Methods: A randomised, double-blind, parallel study was performed in 26 drug-naive type 2 diabetes patients treated with: (1) pioglitazone (n = 14) or (2) aggressive nutritional therapy (n = 12) to reduce HbA(1c) to levels observed in the pioglitazone-treated group. Participants were assigned randomly to treatment using a table of random numbers. Before and after 6 months, patients reported to the Clinical Research Center of the Texas Diabetes Institute for a vastus lateralis muscle biopsy followed by a 180 min euglycaemic-hyperinsulinaemic (80 mU m(-2) min(-1)) clamp.
Results: All patients in the pioglitazone (n = 14) or nutritional therapy (n = 12) group were included in the analysis. Pioglitazone significantly increased plasma adiponectin concentration by 79% and reduced fasting plasma NEFA by 35% (both p < 0.01). Following pioglitazone, insulin-stimulated glucose disposal increased by 30% (p < 0.01), and muscle AMPK and acetyl-CoA carboxylase (ACC) phosphorylation increased by 38% and 53%, respectively (p < 0.05). Pioglitazone increased mRNA levels for adiponectin receptor 1 and 2 genes (ADIPOR1, ADIPOR2), peroxisome proliferator-activated receptor gamma, coactivator 1 gene (PPARGC1) and multiple genes involved in mitochondrial function and fat oxidation. Despite a similar reduction in HbA(1c) and similar improvement in insulin sensitivity with nutritional therapy, there were no significant changes in muscle AMPK and ACC phosphorylation, or the expression of ADIPOR1, ADIPOR2, PPARGC1 and genes involved in mitochondrial function and fat oxidation. No adverse (or unexpected) effects or side effects were reported from the study.
Conclusions/interpretations: Pioglitazone increases plasma adiponectin levels, stimulates muscle AMPK signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation. These changes may represent an important cellular mechanism by which thiazolidinediones improve skeletal muscle insulin sensitivity.
Trial registration: NCT 00816218 FUNDING: This trial was funded by National Institutes of Health Grant DK24092, VA Merit Award, GCRC Grant RR01346, Executive Research Committee Research Award from the University of Texas Health Science Center at San Antonio, American Diabetes Association Junior Faculty Award, American Heart Association National Scientist Development Grant, Takeda Pharmaceuticals North America Grant and Canadian Institute of Health Research Grant.
Trial registration: ClinicalTrials.gov NCT00816218.
Figures
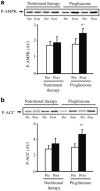
Fig. 1
Effect of pioglitazone and nutritional therapy on AMPK (a) and ACC (b) phosphorylation (P). Data are expressed as arbitrary units (AU). Protein extracts were available for 12 pioglitazone- and nine nutritional therapy-treated patients. Representative blots for two patients are shown. *p<0.05 vs pretreatment; †p<0.05 for the comparison between pioglitazone- and nutritional therapy-treated groups (change from baseline). Means±SEM
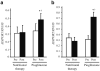
Fig. 2
Effect of pioglitazone and nutritional therapy on mRNA expression of ADIPOR1 (a), and ADIPOR2 (b). Expression data were normalised by dividing the amount of the gene of interest by the amount of RN18S gene used as an internal control. *p<0.05 vs pre-treatment; †p<0.05 for the comparison between pioglitazone- and nutritional therapy-treated groups (change from baseline). Means±SEM
Fig. 3
a Effect of pioglitazone and nutritional therapy on PPARGC1A protein levels. Data are expressed as arbitrary units (AU). Protein extracts were available for 12 pioglitazone- and nine nutritional therapy-treated patients. Representative blots for two patients are shown. b, c Effect of pioglitazone and nutritional therapy on mRNA expression of PPARGC1A (b) and PPARGC1B (c). Expression data were normalised by dividing the amount of the gene of interest by the amount of RN18S gene used as an internal control. *p<0.05 and **p<0.01 vs pretreatment; †p<0.05 for the comparison between pioglitazone- and nutritional therapy-treated groups (change from baseline). Means±SEM
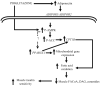
Fig. 4
Proposed mechanism of action of pioglitazone. Pioglitazone increases plasma adiponectin concentration. AMPK and ACC activity is increased following pioglitazone, which is mediated via adiponectin or represents a possible direct effect of pioglitazone on AMPK. Pioglitazone stimulates the expression of PPARGC1A, CPT1B and a number of mRNAs involved in mitochondrial function and NEFA oxidation. P-, phosphorylated; DAG, diacylglycerol; FACoA, fatty acyl CoA
Similar articles
- The effect of muraglitazar on adiponectin signalling, mitochondrial function and fat oxidation genes in human skeletal muscle in vivo.
Coletta DK, Fernandez M, Cersosimo E, Gastaldelli A, Musi N, DeFronzo RA. Coletta DK, et al. Diabet Med. 2015 May;32(5):657-64. doi: 10.1111/dme.12664. Epub 2015 Jan 7. Diabet Med. 2015. PMID: 25484175 Free PMC article. Clinical Trial. - Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus.
Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Bajaj M, et al. Diabetologia. 2007 Aug;50(8):1723-31. doi: 10.1007/s00125-007-0698-9. Epub 2007 May 23. Diabetologia. 2007. PMID: 17520238 Clinical Trial. - Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study.
Tripathy D, Daniele G, Fiorentino TV, Perez-Cadena Z, Chavez-Velasquez A, Kamath S, Fanti P, Jenkinson C, Andreozzi F, Federici M, Gastaldelli A, Defronzo RA, Folli F. Tripathy D, et al. Diabetologia. 2013 Oct;56(10):2153-63. doi: 10.1007/s00125-013-2976-z. Epub 2013 Jun 30. Diabetologia. 2013. PMID: 23811853 Clinical Trial. - Hypoadiponectinaemia in diabetes mellitus type 2: molecular mechanisms and clinical significance.
Su H, Lau WB, Ma XL. Su H, et al. Clin Exp Pharmacol Physiol. 2011 Dec;38(12):897-904. doi: 10.1111/j.1440-1681.2011.05606.x. Clin Exp Pharmacol Physiol. 2011. PMID: 21916932 Free PMC article. Review. - Adiponectin signalling in bone homeostasis, with age and in disease.
Lewis JW, Edwards JR, Naylor AJ, McGettrick HM. Lewis JW, et al. Bone Res. 2021 Jan 7;9(1):1. doi: 10.1038/s41413-020-00122-0. Bone Res. 2021. PMID: 33414405 Free PMC article. Review.
Cited by
- Molecular Implications of the PPARs in the Diabetic Eye.
Ciudin A, Hernández C, Simó R. Ciudin A, et al. PPAR Res. 2013;2013:686525. doi: 10.1155/2013/686525. Epub 2013 Feb 4. PPAR Res. 2013. PMID: 23431285 Free PMC article. - Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents.
Di Pino A, DeFronzo RA. Di Pino A, et al. Endocr Rev. 2019 Dec 1;40(6):1447-1467. doi: 10.1210/er.2018-00141. Endocr Rev. 2019. PMID: 31050706 Free PMC article. Review. - Pioglitazone does not enhance exogenous glucose oxidation or metabolic clearance rate during aerobic exercise in men under acute high-altitude exposure.
Margolis LM, Wilson MA, Drummer DJ, Carrigan CT, Murphy NE, Allen JT, Dawson MA, Mantzoros CS, Young AJ, Pasiakos SM. Margolis LM, et al. Am J Physiol Regul Integr Comp Physiol. 2024 Jul 1;327(1):R25-R34. doi: 10.1152/ajpregu.00064.2024. Epub 2024 Apr 29. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38682243 Free PMC article. Clinical Trial. - Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: Current status.
Stein LL, Dong MH, Loomba R. Stein LL, et al. Adv Ther. 2009 Oct;26(10):893-907. doi: 10.1007/s12325-009-0072-z. Epub 2009 Nov 16. Adv Ther. 2009. PMID: 19921118 Free PMC article. Review. - Role of lipotoxicity in endothelial dysfunction.
Kim JA, Montagnani M, Chandrasekran S, Quon MJ. Kim JA, et al. Heart Fail Clin. 2012 Oct;8(4):589-607. doi: 10.1016/j.hfc.2012.06.012. Epub 2012 Aug 10. Heart Fail Clin. 2012. PMID: 22999242 Free PMC article. Review.
References
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:178–269.
- Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37:595–607. - PubMed
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK024092/DK/NIDDK NIH HHS/United States
- R01 DK067690/DK/NIDDK NIH HHS/United States
- DK24092/DK/NIDDK NIH HHS/United States
- R56 DK024092/DK/NIDDK NIH HHS/United States
- M01 RR001346/RR/NCRR NIH HHS/United States
- R01 DK079195/DK/NIDDK NIH HHS/United States
- RR01346/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous