Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation - PubMed (original) (raw)
Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation
Bin Zheng et al. Mol Cell. 2009.
Abstract
The LKB1-AMPK signaling pathway serves as a critical cellular sensor coupling energy homeostasis to cell growth, proliferation, and survival. However, how tumor cells suppress this signaling pathway to gain growth advantage under conditions of energy stress is largely unknown. Here, we show that AMPK activation is suppressed in melanoma cells with the B-RAF V600E mutation and that downregulation of B-RAF signaling activates AMPK. We find that in these cells LKB1 is phosphorylated by ERK and Rsk, two kinases downstream of B-RAF, and that this phosphorylation compromises the ability of LKB1 to bind and activate AMPK. Furthermore, expression of a phosphorylation-deficient mutant of LKB1 allows activation of AMPK and inhibits melanoma cell proliferation and anchorage-independent cell growth. Our findings provide a molecular linkage between the LKB1-AMPK and the RAF-MEK-ERK pathways and suggest that suppression of LKB1 function by B-RAF V600E plays an important role in B-RAF V600E-driven tumorigenesis.
Figures
Figure 1
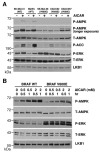
B-RAF V600E suppresses AMPK activity. (A) Phosphorylation of AMPK and ACC in human melanoma cells containing WT B-RAF or V600E mutant. Cells were treated with or without 1 mM AICAR for 1 hr. Cell lysates were used for western blotting with indicated antibodies. (B) Expression of B-RAF V600E attenuates AMPK activation in C140 melanocytes. C140 stably expressed B-RAF WT or V600E mutant were treated with indicated concentration of AICAR.
Figure 2
Down-regulation of B-RAF signaling activates AMPK. (A) Knockdown of B-RAF expression by RNA interference activates AMPK. SK-Mel-28 cells were infected with retrovirus containing two different shRNA constructs in pSUPER-retro against B-RAF or pSUPER-retro empty vector. (B) Knockdown of MEK1 expression by RNA interference activates AMPK. SK-Mel-28 cells were infected with retrovirus containing two different shRNA constructs in pSM2C against MEK1 or control empty vector. (C) Induction of AMPK phosphorylation by various inhibitors against the RAF-MEK-ERK signaling cascade. SK-MEL-28 cells were treated with DMSO, 20 μM U0126, or 50 μM PD98059 for 1 hr.
Figure 3
Activation of AMPK by U0126 is dependent on the presence of LKB1. (A) U0126-induced activation of AMPK is dependent on LKB1 in MEFs. Immortalized Lkb1+/+ and _Lkb1_-/- MEFs were serum-starved and treated with 20 μM of U0126 for 2 hr. (B) U0126-induced activation of AMPK is dependent on LKB1 in SK-Mel-28 cells. SK-Mel-28 cells were infected with lentivirus encoding two different shRNA against LKB1 (sh2 and sh3) or control shRNA (sh1), serum-starved and treated with 20 μM of U0126 for 2 hr.
Figure 4
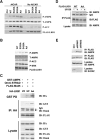
Phosphorylation of Ser325 and Ser428 of LKB1 by two downstream kinases of B-RAF, ERK and p90Rsk, respectively. (A) Identification of phosophorylated LKB1 peptides containing Ser325 and Ser428 by LC-MS/MS analysis. HEK293 cells transfected with FLAG-LKB1 were serum-starved and pretreated with or without 20 μM of U0126 for 2 hr before the addition of 200 nM PMA for 20 min. FLAG-LKB1 proteins were immunoprecipitated using anti-FLAG M2 agarose beads and subjected to trypsin or chymotrypsin digestion followed by the LC-MS/MS analysis. (B) Inhibition of LKB1 Ser325 and Ser428 phosphorylation by MEK inhibitors U0126 and PD98059. Cell lysates from SK-Mel-28 stably expressing FLAG-LKB1 were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies. Numbers indicate relative intensity as quantified by image J analysis. (C) Attenuation of LKB1 Ser325 and Ser428 phosphorylation upon knockdown of B-RAF expression. SK-Mel-28 cells stably expressing FLAG-LKB1 were infected with retrovirus containing two different shRNA constructs in pSUPER-retro against B-RAF or pSUPER-retro empty vector. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies. Numbers indicate relative intensity as quantified by image J analysis. (D) ERK directly phosphoryates LKB1 in vitro. GST-LKB1 (D194A) proteins were expressed in E. coli, purified and incubated with active recombinant ERK proteins in the presence of γ-32P-ATP. Autoradiography was performed. (E) Ser325 is critical for phosphorylation of LKB1 by ERK in vitro. HA-LKB1 WT and S325A mutant were immunoprecipitated from HEK293 cells and incubated with recombinant ERK proteins. Protein from the assays and HEK293 cell lysates were used for western blotting analysis with phospho-S325 LKB1 antibody and HA antibody, respectively. (F) HA-LKB1 coimmunoprecipitates with FLAG-ERK2. HEK293 cells were transfected with HA-LKB1 together with FLAG-ERK2 wildtype or kinase dead mutant. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting with HA antibody. (G) HA-ERK2 coimmunoprecipitates with FLAG-LKB1-N, but no FLAG-LKB1-C. Cos-7 cells were transfected with HA-ERK2 together with FLAG-LKB1 full-length (FL), N (a.a 1-309), C (a.a.310-433) or control vector. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting with HA antibody. (H) HA-LKB1 coimmunoprecipitates with B-RAF V600E, but not WT B-RAF. HEK293 cells were transfected with FLAG-BRAF, HA-LKB1 or empty vectors as indicated. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by immunoblotting with indicated antibodies. (I) Expression of BRAF V600E enhances the association between LKB1 and ERK. HEK293 cells were transfected with FLAG-LKB1, HA-ERK together with control vector, FLAG-B-RAF WT or B-RAF V600E constructs as indicated. Cell lysates were immunoprecipitated with anti-HA antibodies followed by immunoblotting with indicated antibodies.
Figure 5
Phosphorylation of LKB1 on Ser325 and Ser428 is involved in the regulation of AMPK activation by LKB1. (A) Mutation of Ser325 or Ser428 of LKB1 to Ala enhances its activity on AMPK activation. _Lkb1_-/- MEFs were infected with retrovirus containing the vector control, WT LKB1, S325A, S428A or S325A/S428A LKB1, and treated with 1 mM AICAR for 1 hr. Cell lysates were used for western blotting with indicated antibodies. (B) Expression of LKB1 S325A/S428A mutant stimulates AMPK in SK-MEL-28 cells. SK-MEL-28 cells were infected with retrovirus containing WT LKB1, S428A, S325A or S325A/S428A (AA) LKB1 mutants. Cell lysates were used for western blotting analysis with indicated antibodies. (C) Mutation of Ser325 and Ser428 to Ala enhances the ability of LKB1 to associate with AMPK, but not STRAD and MO25. HEK293 cells were transfected with GST-AMPKα1, Omni-STRAD, FLAG-MO25 together with WT or S325A/S428A (AA) mutant of HA-LKB1. Cell lysates were immunoprecipitated with indicated antibodies or incubated with GSH agarose beads for 2hr followed by western blotting with indicated antibodies. (D) Mutation of Ser325 and Ser428 to alanines or U0126 treatment enhances the ability of LKB1 to associate with endogenous AMPK in SK-Mel-28 cells. SK-Mel-28 cells stably expressing FLAG-LKB1 WT or AA mutant were treated with DMSO or 20 μM of U0126 for 2 hr. Cell lysates were incubated with M2 anti-FLAG agarose beads for 2 hr followed by Western blotting with indicated antibodies. (E) Knockdown of B-RAF expression enhances the ability of LKB1 to associate with endogenous AMPK in SK-Mel-28 cells. Sk-Mel-28 cells stably expressing FLAG-LKB1 WT were infected with retrovirus containing two different shRNA constructs in pSUPER-retro against B-RAF or pSUPER-retro empty vector. Cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies.
Figure 6
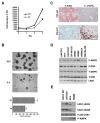
Phosphorylation of LKB1 on Ser325 and Ser428 are critical for cell proliferation and anchorage-independent growth. (A) Expression of LKB1 S325A/S428A (AA) mutant inhibits cell proliferation. Cell proliferation curves of SK-MEL-28 cells stably expressing WT LKB1 or AA mutant were measured. One representative from three independent experiments is shown. (B) Expression of LKB1 S325A/S428A (AA) mutant inhibits cell transformation. SK-MEL-28 cells stably expressing LKB1 WT LKB1 or AA mutant were used in sofa agar assay. Colonies were photographed after 28 days of growth. Error bars indicate SD. One representative from three independent experiments is shown. (C) Inverse correlation between phospho-ERK and phospho-AMPK activities in human melanoma tumor samples. Representative images of human melanoma tumor samples stained with phospho-AMPK or phospho-ERK antibodies in immunohistochemical analysis. (D) Inverse correlation between phospho-ERK and phospho-AMPK activities in human melanoma cells containing B-RAF V600E mutation. Total cell lysates from several human melanoma cell lines were used in Western blotting analysis with indicated antibodies. (E) Phosphorylation levels of LKB1 Ser325 and Ser428 in human melanoma cells containing B-RAF V600E mutation. Various melanoma cell lines were stably transfected with pBabe-FLAG-LKB1 WT, and cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads followed by western blotting using indicated antibodies.
Figure 7
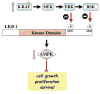
A model for negative regulation of LKB1/AMPK activity by BRAF V600E signaling.
Similar articles
- Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF.
Esteve-Puig R, Canals F, Colomé N, Merlino G, Recio JA. Esteve-Puig R, et al. PLoS One. 2009;4(3):e4771. doi: 10.1371/journal.pone.0004771. Epub 2009 Mar 10. PLoS One. 2009. PMID: 19274086 Free PMC article. - B-Raf(V600E) signaling deregulates the mitotic spindle checkpoint through stabilizing Mps1 levels in melanoma cells.
Cui Y, Guadagno TM. Cui Y, et al. Oncogene. 2008 May 15;27(22):3122-33. doi: 10.1038/sj.onc.1210972. Epub 2007 Dec 10. Oncogene. 2008. PMID: 18071315 - ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells.
Cesi G, Walbrecq G, Zimmer A, Kreis S, Haan C. Cesi G, et al. Mol Cancer. 2017 Jun 8;16(1):102. doi: 10.1186/s12943-017-0667-y. Mol Cancer. 2017. PMID: 28595656 Free PMC article. - Controlling the master-upstream regulation of the tumor suppressor LKB1.
Kullmann L, Krahn MP. Kullmann L, et al. Oncogene. 2018 Jun;37(23):3045-3057. doi: 10.1038/s41388-018-0145-z. Epub 2018 Mar 15. Oncogene. 2018. PMID: 29540834 Review. - Targeting oncogenic Raf protein-serine/threonine kinases in human cancers.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2018 Sep;135:239-258. doi: 10.1016/j.phrs.2018.08.013. Epub 2018 Aug 15. Pharmacol Res. 2018. PMID: 30118796 Review.
Cited by
- Dysregulation of mTOR activity through LKB1 inactivation.
Zhou W, Marcus AI, Vertino PM. Zhou W, et al. Chin J Cancer. 2013 Aug;32(8):427-33. doi: 10.5732/cjc.013.10086. Epub 2013 May 14. Chin J Cancer. 2013. PMID: 23668926 Free PMC article. Review. - RSK regulates activated BRAF signalling to mTORC1 and promotes melanoma growth.
Romeo Y, Moreau J, Zindy PJ, Saba-El-Leil M, Lavoie G, Dandachi F, Baptissart M, Borden KLB, Meloche S, Roux PP. Romeo Y, et al. Oncogene. 2013 Jun 13;32(24):2917-2926. doi: 10.1038/onc.2012.312. Epub 2012 Jul 16. Oncogene. 2013. PMID: 22797077 Free PMC article. - Pseudomonas aeruginosa lectin LecB impairs keratinocyte fitness by abrogating growth factor signalling.
Landi A, Mari M, Kleiser S, Wolf T, Gretzmeier C, Wilhelm I, Kiritsi D, Thünauer R, Geiger R, Nyström A, Reggiori F, Claudinon J, Römer W. Landi A, et al. Life Sci Alliance. 2019 Nov 15;2(6):e201900422. doi: 10.26508/lsa.201900422. Print 2019 Dec. Life Sci Alliance. 2019. PMID: 31732693 Free PMC article. - Regulation of oxysterol-binding protein Golgi localization through protein kinase D-mediated phosphorylation.
Nhek S, Ngo M, Yang X, Ng MM, Field SJ, Asara JM, Ridgway ND, Toker A. Nhek S, et al. Mol Biol Cell. 2010 Jul 1;21(13):2327-37. doi: 10.1091/mbc.e10-02-0090. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444975 Free PMC article. - AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function.
Hardie DG. Hardie DG. Genes Dev. 2011 Sep 15;25(18):1895-908. doi: 10.1101/gad.17420111. Genes Dev. 2011. PMID: 21937710 Free PMC article. Review.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. - PubMed
- Asara JM, Christofk HR, Freimark LM, Cantley LC. A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics. 2008;8:994–999. - PubMed
- Ashrafian H. Cancer's sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet. 2006;367:618–621. - PubMed
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. - PubMed
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99CA133245/CA/NCI NIH HHS/United States
- K99 CA133245/CA/NCI NIH HHS/United States
- P01 CA120964/CA/NCI NIH HHS/United States
- CA102694/CA/NCI NIH HHS/United States
- K99 CA133245-01/CA/NCI NIH HHS/United States
- U01 CA084313/CA/NCI NIH HHS/United States
- R01 CA093947/CA/NCI NIH HHS/United States
- U01 CA84313/CA/NCI NIH HHS/United States
- R01 CA093947-08/CA/NCI NIH HHS/United States
- R01 GM056203-12/GM/NIGMS NIH HHS/United States
- R01 CA93947/CA/NCI NIH HHS/United States
- GM56203/GM/NIGMS NIH HHS/United States
- P01 CA120964-01A10002/CA/NCI NIH HHS/United States
- R01 GM056203/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous