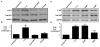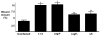Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling - PubMed (original) (raw)
Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling
Toni A Nagy et al. J Infect Dis. 2009.
Abstract
Helicobacter pylori is the strongest identified risk factor for gastric adenocarcinoma. One H. pylori virulence constituent that augments cancer risk is the cag secretion system, which translocates CagA and peptidoglycan into host cells, eventuating in activation of signal transduction pathways. AKT is a target of phosphatidylinositol 3-kinase (PI3K) and is activated in gastric cancer, but the relationship between PI3K-AKT and H. pylori-induced cellular responses with carcinogenic potential remains unclear. We defined the molecular pathways mediating H. pylori-stimulated AKT activation and the biological consequences of these events in gastric epithelial cells. H. pylori enhanced PI3K-AKT signaling in a Src- and epidermal growth factor receptor-dependent manner, which was also mediated by a functional cag secretion system and peptidoglycan. PI3K activation attenuated apoptosis in response to infection and was required for H. pylori-induced cell migration. These results indicate that PI3K-AKT signaling regulates pathophysiologic responses to H. pylori that may lower the threshold for carcinogenesis.
Conflict of interest statement
There are no conflicts of interest for any of the authors in regard to this manuscript.
Figures
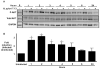
Figure 1. H. pylori induces AKT activation in vitro in a time-dependent manner
(A) AGS cells were co-cultured with the H. pylori cag+ strain 7.13 at a bacteria/cell ratio of 100:1. One through twenty-four hours after incubation, whole cell lysates were harvested and subjected to Western blot analysis using an anti-phospho-AKT antibody. (-), cells incubated with medium alone. A representative blot is shown. Western blots for total AKT served as normalization controls for AGS cell viability under different experimental conditions and Western blots for GAPDH served as loading controls. (B) Densitometric analysis of multiple Western blot repetitions performed on at least 3 occasions. Levels of phospho-AKT were normalized to total AKT and levels were expressed as fold-induction of infected cells compared with uninfected cells at each time point. Error bars = SEM. *P < 0.01 vs. uninfected control.
Figure 2. AKT phosphorylation by H. pylori is dependent on specific genes within the cag pathogenicity island
(A) AGS cells were incubated in the absence or presence of live H. pylori strain 7.13 at a bacteria/cell ratio of 100:1, heat-killed H. pylori, or H. pylori 7.13 filtrate for two hours. Whole cell lysates were subjected to Western blot analysis using an anti-phospho AKT antibody. Anti-total AKT blots served as normalization controls for AGS cell viability under different experimental conditions and anti-GAPDH blots served as loading controls. (B) Densitometric analysis of Western blots performed on 3 occasions. Error bars = SEM. *P <0.04 vs. AGS cells alone. (C) AGS cells were cultured in the absence or presence of the H. pylori cag+ strain 7.13 or its isogenic cagA- or cagE- null mutant derivatives at bacteria/cell ratios of 100:1. Two hours post infection, whole cell lysates were subjected to Western blot analysis using an anti-phospho-AKT antibody. A representative blot is shown. Western blots for total AKT served as normalization controls and Western blots for GAPDH served as loading controls. (D) Densitometric analysis of multiple Western blot repetitions performed on at least 5 occasions. Error bars = SEM. *P < 0.002 vs. AGS cells alone.
Figure 3. AKT phosphorylation by H. pylori is mediated by peptidoglycan
(A) AGS cells were cultured in the absence or presence of wild-type H. pylori strain 7.13 or its isogenic cagA- or slt- null mutant at a bacteria/cell ratio of 100:1. Two hours post infection, whole-cell lysates were subjected to Western blot analysis using an anti-phospho-tyrosine 99 antibody or an anti-CagA antibody. A representative blot is shown. Western blots for GAPDH served as loading controls. (B) H. pylori strain 7.13 or its isogenic slt null mutant derivative, were added to AGS cells at a bacteria/cell ratio of 100:1. Two hours after incubation, whole-cell lysates were subjected to Western blot analysis using an anti-phospho-AKT antibody. A representative blot is shown. Western blots for total AKT served as normalization controls for AGS cell viability under different experimental conditions and Western blots for GAPDH served as loading controls. (C) Densitometric analysis of multiple Western blot repetitions performed on at least 3 occasions. Error bars = SEM. *P < 0.04 vs AGS cells alone; **P < 0.009 vs AGS cells incubated with wild-type H. pylori.
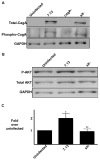
Figure 4. _H. pylori_-induced AKT phosphorylation in AGS cells is dependent on activation of PI3K, EGFR, and Src
(A)_H. pylori strain 7.13 was added to AGS cells at a bacteria/cell concentration of 100:1. Two hours post-infection, whole cell lysates were subjected to Western blot analysis using an anti-phospho-Src or an anti-phospho-EGFR antibody. (-), cells incubated with medium alone. A representative blot is shown. Western blots for total Src or EGFR served as normalization controls for AGS cell viability under different experimental conditions. (B) AGS cells were incubated with the PI3K inhibitor LY294002 (50 μmol/L), EGFR kinase inhibitor AG1478 (600 nmol/L), or Src inhibitor PP2 (10 μmol/L) for one hour prior to EGF exposure for 15 minutes. Levels of phospho- and total AKT were determined by Western blot analysis of whole cell lysates. (C) H. pylori strain 7.13 was added to AGS cells at a bacteria/cell concentration of 100:1 in the absence or presence of vehicle alone (DMSO), or 50 μmol/L LY294002, 600 nmol/L AG1478, or 10 μmol/L PP2. Two hours post-infection, whole cell lysates were subjected to Western blot analysis using an anti-phospho-AKT antibody. (-), cells incubated with medium alone. A representative blot is shown. Western blots for total AKT served as normalization controls for AGS cell viability under different experimental conditions and Western blots for GAPDH served as loading controls. (D) Densitometric analysis of multiple Western blot repetitions performed on at least 3 occasions. Error bars = SEM. *P < 0.0001 vs. AGS cells alone. (E) H. pylori strain 7.13 was added to AGS cells at a bacteria/cell concentration of 100:1 in the absence or presence of vehicle alone (DMSO), or 2 μmol/L SU6656, 50 μmol/L AG1295, or 10 μmol/L STI-571. Two hours post-infection, whole cell lysates were subjected to Western blot analysis using an anti-phospho-AKT antibody. (-), cells incubated with medium alone. A representative blot is shown. Western blots for total AKT served as normalization controls for AGS cell viability under different experimental conditions and Western blots for GAPDH served as loading controls. (F) Densitometric analysis of multiple Western blot repetitions performed on at least 3 occasions. Error bars = SEM. *P < 0.04 vs. AGS cells alone. (G) AGS cells were cultured in the absence or presence of the H. pylori cag+ strain 7.13 or its isogenic cagA-, cagE-, or _slt_- null mutant derivatives at bacteria/cell ratios of 100:1. Two hours post infection, whole cell lysates were subjected to Western blot analysis using an anti-phospho-Gab1 antibody. EGF was used as a positive control for Gab1 phosphorylation and was added for 15 minutes. A representative blot is shown. Western blots for total Gab1 served as normalization controls and Western blots for GAPDH served as loading controls. Densitometric analysis of multiple Western blot repetitions performed on at least 3 occasions is shown below representative Western blot. Error bars = SEM. *P < 0.02 vs. AGS cells alone.
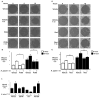
Figure 5. Activation of PI3K-AKT and Rac mediates _H. pylori_-induced cell migration
(A) AGS cells were grown to confluency and incubated with the PI3K inhibitor LY294002 (50 μM) or vehicle alone (DMSO) for one hour. A wound was then introduced into the cell monolayer and medium or H. pylori strain 7.13 was added. Wound areas were measured at zero, six and sixteen hours post-infection. (B) Quantification of wound closure for each treatment group in experiments performed on at least 5 independent occasions. (-), cells incubated without H. pylori. Error bars = SEM. * P < 0.04 vs AGS cells infected with H. pylori strain 7.13 in the presence of the PI3K inhibitor LY294002 at both six and 16 hours. (C) AGS cells were grown to confluency and incubated with 600 nmol/L AG1478, 10 μmol/L PP2, or 50 μmol/L AG1295, or vehicle alone (DMSO) for one hour. A wound was then introduced into the cell monolayer and medium or H. pylori strain 7.13 was added. Wound areas were measured at zero and six hours post-infection. Quantification of wound closure for each treatment group in experiments performed on at least 3 independent occasions is shown. (-), cells incubated without H. pylori. Error bars = SEM. * P < 0.005 vs uninfected AGS cells alone or in the presence of AG1295. (D) AGS cells were grown to confluency and incubated with the Rac1 inhibitor NSC23766 (50 μM) or vehicle alone (water) for one hour. A wound was then introduced into the cell monolayer and medium or H. pylori strain 7.13 was added. Wound areas were measured at zero, six and sixteen hours post-infection. (E) Quantification of wound closure for each treatment group in experiments performed on at least 5 independent occasions. (-), cells incubated without H. pylori. Error bars = SEM. ** P < 0.01 vs AGS cells infected with H. pylori strain 7.13 in the presence of the Rac inhibitor NSC23766 at both six and 16 hours.
Figure 6. _H. pylori_-induced cell migration is dependent on the cag pathogenicity island and peptidoglycan
AGS cells were grown to confluency and a wound was introduced into the monolayer. Medium, H. pylori strain 7.13, or isogenic cagA-, cagE-, or slt- null mutant derivatives were then added at bacteria/cell ratios of 100:1. Wound areas were measured at time zero and six hours post-infection. Quantification of wound closure is shown for each treatment group in experiments performed on at least 3 occasions. Error bars = SEM. * P < 0.007 vs uninfected control; ** P < 0.007 vs AGS cells infected with H. pylori strain 7.13.
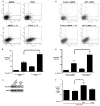
Figure 7. Activation of AKT by H. pylori promotes cell survival
(A) AGS cells were co-cultured with H. pylori strain 7.13 at a bacteria/cell concentration of 100:1, in the absence or presence of the PI3K inhibitor LY294002 (50 μM) or vehicle alone (DMSO) for 24 hours. Live cells were stained with Annexin V-APC and PI, and apoptosis was quantified by flow cytometry. The upper right quadrant represents late apoptosis, and the lower right quadrant represents early apoptosis. (B) Combined percentage of early and late apoptotic cells for experiments performed on at least 5 occasions. (-), cells incubated without H. pylori. Error bars = SEM. * P < 0.005 vs AGS cells infected with H. pylori strain 7.13 at MOI of 100:1 in the presence of vehicle alone. (C) AGS cells were transiently transfected with scrambled or AKT-specific siRNA, total protein was extracted and subjected to Western blot analysis using an anti-AKT antibody. (D) AGS cells transiently transfected with control or AKT-specific siRNA were co-cultured with H. pylori strain 7.13 at a bacteria/cell concentration of 100:1 for 24 hours. Live cells were stained with Annexin V-APC and PI, and apoptosis was quantified by flow cytometry. The upper right quadrant represents late apoptosis, and the lower right quadrant represents early apoptosis. (E) Combined percentage of early and late apoptotic cells for experiments performed on at least 3 occasions. (-), cells incubated without H. pylori. Error bars = SEM. * P < 0.05 vs AKT siRNA-treated AGS cells infected with H. pylori strain 7.13. (F) AGS cells were co-cultured with or without H. pylori strain 7.13 at a bacterial/cell concentration of 100:1, treated with LY294002 (50μM) or medium alone, and then exposed to Staurosporine (Stsp). Cells were then stained with Annexin V-APC and PI, and subjected to flow cytometry. (-), cells incubated without H. pylori. ** P< 0.05 vs AGS cells infected with H. pylori strain 7.13 in the presence of Stsp alone.
Similar articles
- Helicobacter pylori-associated regulation of forkhead transcription factors FoxO1/3a in human gastric cells.
Tabassam FH, Graham DY, Yamaoka Y. Tabassam FH, et al. Helicobacter. 2012 Jun;17(3):193-202. doi: 10.1111/j.1523-5378.2012.00939.x. Epub 2012 Mar 20. Helicobacter. 2012. PMID: 22515357 Free PMC article. - Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation.
Tabassam FH, Graham DY, Yamaoka Y. Tabassam FH, et al. Cell Microbiol. 2009 Jan;11(1):70-82. doi: 10.1111/j.1462-5822.2008.01237.x. Epub 2008 Sep 8. Cell Microbiol. 2009. PMID: 18782353 Free PMC article. - Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis.
Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM Jr, Cover TL, Washington MK, Wilson KT, Polk DB. Yan F, et al. Gastroenterology. 2009 Apr;136(4):1297-1307, e1-3. doi: 10.1053/j.gastro.2008.12.059. Epub 2009 Jan 1. Gastroenterology. 2009. PMID: 19250983 Free PMC article. - Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways.
Yong X, Tang B, Li BS, Xie R, Hu CJ, Luo G, Qin Y, Dong H, Yang SM. Yong X, et al. Cell Commun Signal. 2015 Jul 11;13:30. doi: 10.1186/s12964-015-0111-0. Cell Commun Signal. 2015. PMID: 26160167 Free PMC article. Review. - Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA.
Peek RM Jr. Peek RM Jr. Sci STKE. 2005 Mar 29;2005(277):pe14. doi: 10.1126/stke.2772005pe14. Sci STKE. 2005. PMID: 15798102 Review.
Cited by
- Exploring the Relationship Between Helicobacter pylori Infection and Biliary Diseases: A Comprehensive Analysis Using the United States National Inpatient Sample (2016-2020).
Ahmad SO, AlAmr M, Taftafa A, AlMazmomy AM, Alkahmous N, Alharran AM, Almarri AM, Alyaqout F, Saad AR, Alazmi AM, Alharran YM, Abotela M, Abu-Zaid A. Ahmad SO, et al. Cureus. 2024 May 28;16(5):e61238. doi: 10.7759/cureus.61238. eCollection 2024 May. Cureus. 2024. PMID: 38939288 Free PMC article. - Induction of COX-2 expression by Helicobacter pylori is mediated by activation of epidermal growth factor receptor in gastric epithelial cells.
Sierra JC, Hobbs S, Chaturvedi R, Yan F, Wilson KT, Peek RM Jr, Polk DB. Sierra JC, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Jul 15;305(2):G196-203. doi: 10.1152/ajpgi.00495.2012. Epub 2013 May 16. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23681474 Free PMC article. - Induction of Fibroblast Growth Factor Receptor 4 by Helicobacter pylori via Signal Transducer and Activator of Transcription 3 With a Feedforward Activation Loop Involving SRC Signaling in Gastric Cancer.
Zhang X, Soutto M, Chen Z, Bhat N, Zhu S, Eissmann MF, Ernst M, Lu H, Peng D, Xu Z, El-Rifai W. Zhang X, et al. Gastroenterology. 2022 Sep;163(3):620-636.e9. doi: 10.1053/j.gastro.2022.05.016. Epub 2022 May 17. Gastroenterology. 2022. PMID: 35588797 Free PMC article. - Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase.
Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM, Delgado AG, Schneider BG, Israel DA, Romero-Gallo J, Nagy TA, Morgan DR, Murray-Stewart T, Bravo LE, Peek RM Jr, Fox JG, Woster PM, Casero RA Jr, Correa P, Wilson KT. Chaturvedi R, et al. Oncogene. 2015 Jun;34(26):3429-40. doi: 10.1038/onc.2014.273. Epub 2014 Sep 1. Oncogene. 2015. PMID: 25174398 Free PMC article. - Helicobacter pylori Infection Activates the Akt-Mdm2-p53 Signaling Pathway in Gastric Epithelial Cells.
Shu X, Yang Z, Li ZH, Chen L, Zhou XD, Xie Y, Lu NH. Shu X, et al. Dig Dis Sci. 2015 Apr;60(4):876-86. doi: 10.1007/s10620-014-3470-2. Epub 2014 Dec 6. Dig Dis Sci. 2015. PMID: 25480405
References
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev Cancer. 2002;2:28–37. - PubMed
- Backert S, Ziska E, Brinkmann V, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cellular Microbiology. 2000;2:155–164. - PubMed
- Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–500. - PubMed
- Higashi H, Tsutsumi R, Muto S, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–6. - PubMed
- Selbach M, Moese S, Hauck CR, Meyer TF, Backert S. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 58587/DK/NIDDK NIH HHS/United States
- R01 CA077955/CA/NCI NIH HHS/United States
- R01 DK058587-08/DK/NIDDK NIH HHS/United States
- R01 DK058587-07/DK/NIDDK NIH HHS/United States
- R01 DK073902-03/DK/NIDDK NIH HHS/United States
- R01 CA077955-10/CA/NCI NIH HHS/United States
- K01 DK077956/DK/NIDDK NIH HHS/United States
- R29 CA077955/CA/NCI NIH HHS/United States
- DK 53902/DK/NIDDK NIH HHS/United States
- R01 CA077955-11/CA/NCI NIH HHS/United States
- R01 DK073902/DK/NIDDK NIH HHS/United States
- K01 DK077956-03/DK/NIDDK NIH HHS/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
- CA 77955/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous