NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation - PubMed (original) (raw)
NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation
Xin-Jian He et al. Genes Dev. 2009.
Abstract
RNA-directed DNA methylation (RdDM) is an RNAi-based mechanism for establishing transcriptional gene silencing in plants. The plant-specific RNA polymerases IV and V are required for the generation of 24-nucleotide (nt) siRNAs and for guiding sequence-specific DNA methylation by the siRNAs, respectively. However, unlike the extensively studied multisubunit Pol II, our current knowledge about Pol IV and Pol V is restricted to only the two largest subunits NRPD1a/NRPD1 and NRPD1b/NRPE1 and the one second-largest subunit NRPD2a. It is unclear whether other subunits may be required for the functioning of Pol IV and Pol V in RdDM. From a genetic screen for second-site suppressors of the DNA demethylase mutant ros1, we identified a new component (referred to as RDM2) as well as seven known components (NRPD1, NRPE1, NRPD2a, AGO4, HEN1, DRD1, and HDA6) of the RdDM pathway. The differential effects of the mutations on two mechanistically distinct transcriptional silencing reporters suggest that RDM2, NRPD1, NRPE1, NRPD2a, HEN1, and DRD1 function only in the siRNA-dependent pathway of transcriptional silencing, whereas HDA6 and AGO4 have roles in both siRNA-dependent and -independent pathways of transcriptional silencing. In the rdm2 mutants, DNA methylation and siRNA accumulation were reduced substantially at loci previously identified as endogenous targets of Pol IV and Pol V, including 5S rDNA, MEA-ISR, AtSN1, AtGP1, and AtMU1. The amino acid sequence of RDM2 is similar to that of RPB4 subunit of Pol II, but we show evidence that RDM2 has diverged significantly from RPB4 and cannot function in Pol II. An association of RDM2 with both NRPD1 and NRPE1 was observed by coimmunoprecipitation and coimmunolocalization assays. Our results show that RDM2/NRPD4/NRPE4 is a new component of the RdDM pathway in Arabidopsis and that it functions as part of Pol IV and Pol V.
Figures
Figure 1.
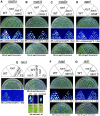
Luminescence and kanamycin resistance phenotypes of ros1 suppressor mutations in known RdDM components. Wild type, ros1, and the double mutants ros1rdm5 (A), ros1rdm6-1 (B), ros1rdm7 (C), ros1rdm8 (D), ros1rdm9 (E), ros1rdm10 (F), and ros1rdm11 (G) were either grown on MS plates for 7–10 d and luminescence was imaged after cold treatment (1 d, 4°C), or were grown on MS plates supplemented with 50 μg/mL kanamycin and the pictures were taken after 1–2 wk. Because the ros1rdm9 mutant is sterile, the F2 progenies from a cross between ros1rdm9 and ros1, instead of ros1rdm9, were grown on plates for phenotype assay. The luminescence of ros1rdm9 was also assayed using NaCl-treated leaves from soil-grown plants. Because the rdm5, rdm6, rdm7, rdm8, rdm9, rdm10, and rdm11 mutants were identified as nrpd1a, nrpd1b, nrpd2a, ago4, hen1, hda6, and drd1, respectively, the names of these mutants are labeled on the top of each panel.
Figure 2.
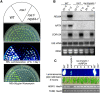
TGS is suppressed in the ros1nrpd4-1 mutant plants. (A) Luminescence and kanamycin resistance phenotypes. Wild type, ros1, and ros1nrpd4-1 were grown on MS plates and imaged after cold treatment (4°C, 24 h). Wild type, ros1, and ros1nrpd4-1 were grown on MS plates with kanamycin (50 μg/mL), and the pictures were taken after 1 wk. (B) The transcript levels of endogenous RD29A and NPTII transgene in wild type, ros1, and ros1nrpd4-1. Total RNA was extracted from 2-wk-old seedlings with or without cold treatment (24 h, 4°C). COR15A and 18S rRNA were used as cold treatment control and RNA loading control, respectively. (C) Assay for complementation of the ros1nrpd4-1 mutant. The plant expression vector PMDC164 harboring NRPD4 genomic sequence was transformed into the ros1nrpd4-1 mutant plants. The leaves from wild type, ros1, ros1nrpd4-1, and the six T1 individual transgenic lines were used for luminescence imaging after treatment with 200 mM NaCl for 3 h. After the genome DNA was digested with the methylation-sensitive enzyme HaeIII, the amplification of AtSN1 in the six transgenic lines was restored to the same level as that in wild type and ros1.
Figure 3.
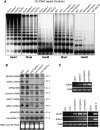
Effect of nrpd4 mutations on DNA methylation. The percentage of cytosine methylation at transgene (A) and endogenous (B) RD29A promoters, and at AtSN1 (C) and MEA-ISR (D) were determined by bisulfite sequencing. The ros1nrpd1a or nrpd1b mutants were used as the mutant controls. (H) A, T, or C. The percentage of cytosine methylation on CG, CHG, and CHH sites is shown. (E) PCR assay of the effect of the nrdp4-1 mutation on DNA methylation of AtSN1. Amplification of AtSN1 was performed after the genomic DNA was digested with the methylation-sensitive restriction enzyme HaeIII. The undigested genomic DNA was amplified as a control. (F) Effect of the nrpd4 mutation on DNA methylation of AtMU1. Genomic DNA from Col-0, nrpd4-2, nrpd1a, and nrpd1b was digested with the methylation-sensitive restriction enzyme HaeIII, followed by Southern hybridization.
Figure 4.
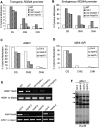
Effect of nrpd4 mutations on DNA methylation, siRNA accumulation, and RNA transcript levels. (A) The nrpd4 mutations reduce DNA methylation at 5S rDNA repeats. Genomic DNA from the indicated genotypes was digested with HpaII (for CG and CHG methylation), MspI (for CHG methylation), and HaeIII (for CHH methylation), followed by Southern hybridization. (B) Detection of RD29A promoter siRNAs, AtSN1 siRNA, siRNA1003, Cluster4 siRNA, AtGP1 siRNA and AtMU1 siRNA, ta-siRNA255, and microRNA171 in the indicated genotypes. The ethidium bromide-stained gel corresponding to tRNA and 5S rRNA was used as a loading control. The positions of size markers are indicated (24 nt or 21 nt). (C) The nrpd4 mutations increase the expression of AtSN1, AtGP1, and AtMU1. The transcript level of the transposons was detected by RT–PCR. TUB8 was used as a control.
Figure 5.
NRPD4 encodes a protein with sequence similarity to RPB4. (A) Diagram of the NRPD4 gene showing the positions of exons (solid boxes), introns, and sites of T-DNA insertions. (B) Sequence alignment of NRPD4 from Arabidopsis and rice and RPB4 from Arabidopsis, rice, yeast, Drosopila, and human. (C) Phylogenetic relationships among NRPD4 and RPB4 proteins. The NRPD4 proteins are from Arabidopsis, rice, and grape, while the RPB4 proteins are from the three plant species and budding yeast, Drosophila, and human. (D) Assay for complementation of the yeast rpb4Δ mutant. The cultures from each of the indicated strains were diluted and spotted onto YPD plates and incubated for 2 d at 28°C or 37°C.
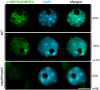
Figure 6.
Subnuclear distribution of NRPD4/NRPE4. Representative images of Arabidopsis interphase nuclei after immunolocalization of NRPD4/NRPE4 (in green) in the wild type (WT) and in the nrpd4/nrpe4 mutant. The frequency of nuclei displaying each interphase pattern is shown on the right. Nuclear DNA is stained with DAPI (blue). Bar, 5 μm.
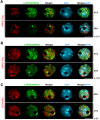
Figure 7.
Colocalization of NRPD4/NRPE4 with Pol IV, Pol V, and AGO4. Immunofluorescence of NRPD4/NRPE4 (green) in transgenic lines expressing full-length epitope-tagged NRPD1 (A), NRPE1 (B), and AGO4 (C) (all in red). The merged images reveal bright yellow signals due to the overlap of red and green channels. The frequency of nuclei displaying each interphase pattern is shown to the right. Nuclear DNA is stained with DAPI (blue). Bar, 5 μm.
Figure 8.
NRPD4/NRPE4 interacts with NRPD1 and NRPE1. Coimmunoprecipitation analysis of the interaction between NRPD4 and NRPD1 (A) or NRPE1 (B). Anti-NRPD4 antibody was incubated with protein extracts from flowers of NRPD1-Flag plants, NRPE1-Flag plants, or wild-type plants without transgenes, followed by immunoprecipitation with protein A beads. The bound proteins were washed and eluted, followed by Western blotting with anti-Flag antibody.
Similar articles
- The RNA silencing enzyme RNA polymerase v is required for plant immunity.
López A, Ramírez V, García-Andrade J, Flors V, Vera P. López A, et al. PLoS Genet. 2011 Dec;7(12):e1002434. doi: 10.1371/journal.pgen.1002434. Epub 2011 Dec 29. PLoS Genet. 2011. PMID: 22242006 Free PMC article. - Distinct and concurrent pathways of Pol II- and Pol IV-dependent siRNA biogenesis at a repetitive trans-silencer locus in Arabidopsis thaliana.
Sasaki T, Lee TF, Liao WW, Naumann U, Liao JL, Eun C, Huang YY, Fu JL, Chen PY, Meyers BC, Matzke AJ, Matzke M. Sasaki T, et al. Plant J. 2014 Jul;79(1):127-38. doi: 10.1111/tpj.12545. Epub 2014 Jun 13. Plant J. 2014. PMID: 24798377 - Metal A and metal B sites of nuclear RNA polymerases Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing.
Haag JR, Pontes O, Pikaard CS. Haag JR, et al. PLoS One. 2009;4(1):e4110. doi: 10.1371/journal.pone.0004110. Epub 2009 Jan 1. PLoS One. 2009. PMID: 19119310 Free PMC article. - Molecular mechanisms of the RNA polymerases in plant RNA-directed DNA methylation.
Xie G, Du X, Hu H, Du J. Xie G, et al. Trends Biochem Sci. 2024 Mar;49(3):247-256. doi: 10.1016/j.tibs.2023.11.005. Epub 2023 Dec 9. Trends Biochem Sci. 2024. PMID: 38072749 Review. - RNA-directed DNA methylation and Pol IVb in Arabidopsis.
Matzke M, Kanno T, Huettel B, Daxinger L, Matzke AJ. Matzke M, et al. Cold Spring Harb Symp Quant Biol. 2006;71:449-59. doi: 10.1101/sqb.2006.71.028. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381327 Review.
Cited by
- RNA Polymerases IV and V Are Involved in Olive Fruit Development.
Serrano A, Moret M, Fernández-Parras I, Bombarely A, Luque F, Navarro F. Serrano A, et al. Genes (Basel). 2023 Dec 19;15(1):1. doi: 10.3390/genes15010001. Genes (Basel). 2023. PMID: 38275583 Free PMC article. - Systematic genome-wide and expression analysis of RNA-directed DNA methylation pathway genes in grapes predicts their involvement in multiple biological processes.
Xiang R, Ahmad B, Liang C, Shi X, Yang L, Du G, Wang L. Xiang R, et al. Front Plant Sci. 2022 Dec 9;13:1089392. doi: 10.3389/fpls.2022.1089392. eCollection 2022. Front Plant Sci. 2022. PMID: 36570893 Free PMC article. - You shall not pass! A Chromatin barrier story in plants.
Velay F, Méteignier LV, Laloi C. Velay F, et al. Front Plant Sci. 2022 Sep 20;13:888102. doi: 10.3389/fpls.2022.888102. eCollection 2022. Front Plant Sci. 2022. PMID: 36212303 Free PMC article. Review. - Multi-omics data integration reveals link between epigenetic modifications and gene expression in sugar beet (Beta vulgaris subsp. vulgaris) in response to cold.
Gutschker S, Corral JM, Schmiedl A, Ludewig F, Koch W, Fiedler-Wiechers K, Czarnecki O, Harms K, Keller I, Martins Rodrigues C, Pommerrenig B, Neuhaus HE, Zierer W, Sonnewald U, Müdsam C. Gutschker S, et al. BMC Genomics. 2022 Feb 17;23(1):144. doi: 10.1186/s12864-022-08312-2. BMC Genomics. 2022. PMID: 35176993 Free PMC article. - AtHDA6 functions as an H3K18ac eraser to maintain pericentromeric CHG methylation in Arabidopsis thaliana.
Wang Q, Bao X, Chen S, Zhong H, Liu Y, Zhang L, Xia Y, Kragler F, Luo M, Li XD, Lam HM, Zhang S. Wang Q, et al. Nucleic Acids Res. 2021 Sep 27;49(17):9755-9767. doi: 10.1093/nar/gkab706. Nucleic Acids Res. 2021. PMID: 34403482 Free PMC article.
References
- Aravin A.A., Sachidanandam R., Girard A., Fejes-Toth K., Hannon G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. - PubMed
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. - PubMed
- Bender J. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 2004;55:41–68. - PubMed
- Chan S.W., Zilberman D., Xie Z., Johansen L.K., Carrington J.C., Jacobsen S.E. RNA silencing genes control de novo DNA methylation. Science. 2004;303:1336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM059138/GM/NIGMS NIH HHS/United States
- R01 GM070795/GM/NIGMS NIH HHS/United States
- R01 GM059138/GM/NIGMS NIH HHS/United States
- R01GM070795/GM/NIGMS NIH HHS/United States
- R01 GM077590/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials