APP binds DR6 to trigger axon pruning and neuron death via distinct caspases - PubMed (original) (raw)
APP binds DR6 to trigger axon pruning and neuron death via distinct caspases
Anatoly Nikolaev et al. Nature. 2009.
Retraction in
- Retraction Note: APP binds DR6 to trigger axon pruning and neuron death via distinct caspases.
Nikolaev A, McLaughlin T, O'Leary DDM, Tessier-Lavigne M. Nikolaev A, et al. Nature. 2024 Jan;625(7993):204. doi: 10.1038/s41586-023-06943-3. Nature. 2024. PMID: 38110576 Free PMC article. No abstract available.
Abstract
Naturally occurring axonal pruning and neuronal cell death help to sculpt neuronal connections during development, but their mechanistic basis remains poorly understood. Here we report that beta-amyloid precursor protein (APP) and death receptor 6 (DR6, also known as TNFRSF21) activate a widespread caspase-dependent self-destruction program. DR6 is broadly expressed by developing neurons, and is required for normal cell body death and axonal pruning both in vivo and after trophic-factor deprivation in vitro. Unlike neuronal cell body apoptosis, which requires caspase 3, we show that axonal degeneration requires caspase 6, which is activated in a punctate pattern that parallels the pattern of axonal fragmentation. DR6 is activated locally by an inactive surface ligand(s) that is released in an active form after trophic-factor deprivation, and we identify APP as a DR6 ligand. Trophic-factor deprivation triggers the shedding of surface APP in a beta-secretase (BACE)-dependent manner. Loss- and gain-of-function studies support a model in which a cleaved amino-terminal fragment of APP (N-APP) binds DR6 and triggers degeneration. Genetic support is provided by a common neuromuscular junction phenotype in mutant mice. Our results indicate that APP and DR6 are components of a neuronal self-destruction pathway, and suggest that an extracellular fragment of APP, acting via DR6 and caspase 6, contributes to Alzheimer's disease.
Figures
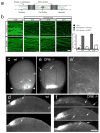
Fig. 1. DR6 regulates degeneration of multiple neuronal classes
(a) Diagram of several TNFR superfamily members possessing Death Domains. (b) DR6 mRNA is expressed by differentiating spinal neurons (including motor (m) and commissural (c)), and by sensory neurons (s) in DRG at E10.5 – E12.5. Expression is low in neuronal progenitors (p) in the ventricular zone (v). (c–f) Anti-DR6.1 (50ug/ml) reduces degeneration in vitro, (c) Anti-DR6.1 inhibits commissural axon degeneration (visualized with GFP, right) and cell body death (TUNEL labeling, left; dots indicate explants) seen after 48hr in dorsal spinal cord cultures, (d, e) Effect on degeneration of sensory (d) or motor (e) axons triggered by trophic deprivation. Axons visualized by immunostaining for tubulin (TuJ1; sensory) or p75NTR (motor), (f) Percent degenerating axon bundles in (c–e) (mean +/− S.E.M.). (g, h) Genetic ablation of DR6 decreases apoptosis in vivo. (g) Immunohistochemistry for cleaved caspase-3 in cervical spinal cord (dh, dorsal horn; vh, ventral horn) and DRG (d) of E15.5 DR6 mutants and heterozygous littermates. (h) Number of positive cells per serial 20 μm section (mean +/− S.E.M.). Scale bar, (c, d): 50 μm; (e): 50 μm; (g, h): 110 μm.
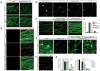
Figure 2. DR6 regulates axon pruning in vitro and in vivo
(a)Diagram of Campenot chamber (adapted from ref. (24)). (b)Local degeneration of sensory axons (TuJ1 immunostain) in Campenot chambers after NGF deprivation from the axonal compartment (top) was delayed by anti-DR6.1 (50 ug/ml) added at time of deprivation (bottom). Right: percent degenerating bundles at 24 and 48hr. (c–j) Compromised pruning of retinal axons in DR6 −/− mice. Dorsal view of (c, e), and vibratome sections through (d, f), the superior colliculus (SC) of wt (c, d) or DR6 −/− (e, f) mice at P6. (c, d) In wild-type, diI-labeled temporal retinal ganglion cell (RGC) axons form a dense termination zone (TZ) in anterior SC (arrowheads: anterior border). Few are outside the immediate TZ area (arrows), (e, f) In DR6 −/− mice, temporal RGC axons and arbors are present in areas far from the TZ (inset, magnified in (e′)) and well posterior to it (arrows). P: posterior; L, lateral; M, medial. Scale bar: (b): 200 μm; 400 μm (c, e); 170 μm (e′); 250 μm (d, f).
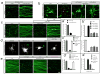
Figure 3. Bax and Caspase-6 regulate axonal degeneration
(a)Local sensory axon degeneration (TuJ1 immunostain) 48hr after NGF deprivation in Campenot chambers was blocked in neurons _from Bax_−/− mice. (b)Dissociated sensory neurons double-labeled for pro-caspase-3 and TuJ1 (left), or pro-caspase-6 and TuJ1 (right). Caspase-3 is detected in cell bodies (arrowheads), caspase-6 in both cell bodies and axons. (c)Local degeneration of sensory axons in Campenot chambers deprived of NGF for 24hr is inhibited by a caspase-6 inhibitor (zVEID-FMK) but not a caspase-3/7 inhibitor (zDEVD-FMK). Quantification in (c′). (d)In dissociated sensory neuron cultures deprived of NGF for 24hr, siRNA knock-down _of caspase_-3 primarily rescues cell body death (TUNEL label), whereas _caspase_-6 knock-down primarily rescues axonal degeneration. Quantification in (d′). Extent of inhibition by individual siRNAs correlates with degree of target knock-down (Supplementary Fig. 6d). (e)Detection of caspase-6 activation in sensory axons with a cleaved caspase-6-specific antibody (left; TuJ1 double-label on right). Punctate activation of caspase-6 following NGF deprivation (16 hr, middle) was reduced by anti-DR6.1 (bottom). (f)Confocal section of a field from (e) shows that actived caspase-6 puncta correspond to sites of tubulin loss (fraction non-overlapping: 79 +/−5%). Scale bar: (a), 250 μml (b): 100 μm; (c): 90 μm; (d): 75 μm; (g): 50 μm; (h): 25 μm.
Figure 4. The amino terminus of APP is a regulated DR6 ligand
(a)Hypothesis: if DR6 is ligand-activated, then DR6-Fc might sequester the ligand and inhibit degeneration. (b)DR6-Fc inhibits local degeneration of sensory axons in Campenot chambers 24hr after NGF deprivation. Quantification in (c). (d, e) DR6-binding sites are lost from axons and released into medium upon trophic deprivation. (d) DR6-AP binding (purple) to _Bax_−/− sensory axons (left) is lost 24hr after NGF deprivation (right), (e) Medium conditioned by sensory axons (in Campenot chambers) or ventral spinal cord explants, maintained with or deprived of trophic factors for 24 hr (sensory: NGF; motor: BDNF and NT3; Bax inhibitor present), was resolved under non-reducing conditions and probed with DR6-AP. (f)Results in (a–e) support a “Ligand Activation” model in which an inactive DR6 surface ligand is shed in active form upon tropic deprivation. (g)APP immunostaining on sections of mouse embryos at indicated ages, showing neuronal and axonal expression, v, ventricular zone; drez, dorsal root entry zone; vlf, ventro-lateral funiculus; s, sensory ganglia; sn, spinal nerve. (h) Domain structure of APP (short form, APP695), indicating beta- and gamma-secretase cleavage sites and antibody binding sites. KPI and OX2: alternatively spliced domains of longer form. Adapted from ref. (20). (i) DR6 binding sites in sensory axon conditioned medium include APP ectodomain fragments. Left: anti-N-APP(poly) detects bands at ~35 kDa (N-APP, major) and ~100 kDa (minor), enriched after trophic deprivation. Middle: anti-sAPPβ detects bands at ~55kDa (major) and ~100 kDa minor). Right: Immunodepletion using anti-N-APP(poly) depletes DR6-AP binding sites. (j) Direct interaction between purified APP [1–286] and DR6-Fc revealed by pull-down. (k) Loss of surface APP in patches from sensory axons 12hr after NGF deprivation is blocked by the BACE inhibitor OM99-2 (10μM) but not the alpha-secretase inhibitor TAPI (20μM). Scale bar: (b): 50 μm; (d): 50 μm; (g): 500 μm; (i) 50 μm.
Figure 5. The APP amino terminus regulates degeneration
(a) Local degeneration of sensory axons in Campenot chambers (NGF deprivation, 24hr) was blocked by anti-N-APP(poly) (20 ug/ml). Quantification in (a′). (b) In dissociated sensory neurons, siRNA knock-down of APP significantly reduces axon degeneration 24hr after trophic deprivation, and partially reduces cell body death. Quantification in (b′). (c)Local degeneration of sensory axons in Campenot chambers (NGF deprivation, 24hr) was inhibited by local addition of BACE inhibitor OM99-2 (10μM). Purified APP[1–286] added locally restored axonal degeneration, an effect inhibited by 50 ng/ml NGF (right). Quantification in (c′). (d)Degeneration of commissural neurons and axons at 48hr was inhibited by anti-N-APP(poly) (20 ug/ml) or BACE inhibitor OM99-2 (10μM), but restored by APP[1–286]. Quantification in (d′). (e) Effect of Abeta antibodies on sensory axon degeneration (NGF deprivation, 24hr). 4G8 partially inhibited; anti- Abeta[17–24] did not. Quantification in (e′). Scale bar: (a, c, e): 50 μm; (b): 40 μm; (d): 200 μm.
Figure 6. APP/DR6 signaling: in vivo evidence, and model
(a) In control (DR6+/−) P0 diaphragm muscle, few axons (green, neurofilament and synaptophysin stain) overshoot endplates (red, fluorescent alpha-bungarotoxin stain), and those that do are short, but in DR6 mutants more overshoot and many are long (arrowheads), (a′) Number overshooting by > 50 μm (this underestimates the effect, since overshooting axons are longer in mutants). Scale bar: 60 μm (left panels), 15 μm (right). (b) The “APP/Death Receptor” mechanism. Trophic deprivation triggers cleavage of surface APP by BACE1, releasing sAPPβ, which is further cleaved by an unknown mechanism (“?”) to release N-APP, which binds DR6 to trigger degeneration through caspase-6 in axons and caspase-3 in cell bodies. Also illustrated is cleavage by gamma secretase to release Abeta and the APP intracellular domain (AICD).
Comment in
- Neuroscience: Good and bad cell death.
Nicholson DW. Nicholson DW. Nature. 2009 Feb 19;457(7232):970-1. doi: 10.1038/457970a. Nature. 2009. PMID: 19225511 No abstract available.
Similar articles
- Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of β-secretase.
Olsen O, Kallop DY, McLaughlin T, Huntwork-Rodriguez S, Wu Z, Duggan CD, Simon DJ, Lu Y, Easley-Neal C, Takeda K, Hass PE, Jaworski A, O'Leary DD, Weimer RM, Tessier-Lavigne M. Olsen O, et al. J Neurosci. 2014 May 7;34(19):6438-47. doi: 10.1523/JNEUROSCI.3522-13.2014. J Neurosci. 2014. PMID: 24806670 Free PMC article. - Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway.
Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Vohra BP, et al. J Neurosci. 2010 Oct 13;30(41):13729-38. doi: 10.1523/JNEUROSCI.2939-10.2010. J Neurosci. 2010. PMID: 20943913 Free PMC article. - Beta amyloid-induced upregulation of death receptor 6 accelerates the toxic effect of N-terminal fragment of amyloid precursor protein.
Xu Y, Wang D, Luo Y, Li W, Shan Y, Tan X, Zhu C. Xu Y, et al. Neurobiol Aging. 2015 Jan;36(1):157-68. doi: 10.1016/j.neurobiolaging.2014.07.027. Epub 2014 Jul 24. Neurobiol Aging. 2015. PMID: 25150572 - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - The molecular bases of Alzheimer's disease and other neurodegenerative disorders.
Maccioni RB, Muñoz JP, Barbeito L. Maccioni RB, et al. Arch Med Res. 2001 Sep-Oct;32(5):367-81. doi: 10.1016/s0188-4409(01)00316-2. Arch Med Res. 2001. PMID: 11578751 Review.
Cited by
- Increased expression of the proapoptotic presenilin associated protein is involved in neuronal tangle formation in human brain.
Yang C, Sun ZP, Jiang J, Cai XL, Wang Y, Wang H, Che C, Tu E, Pan AH, Zhang Y, Wang XP, Cui MZ, Xu XM, Yan XX, Zhang QL. Yang C, et al. Sci Rep. 2024 Oct 25;14(1):25274. doi: 10.1038/s41598-024-77026-0. Sci Rep. 2024. PMID: 39455681 Free PMC article. - Exploring caspase functions in mouse models.
Svandova E, Vesela B, Janeckova E, Chai Y, Matalova E. Svandova E, et al. Apoptosis. 2024 Aug;29(7-8):938-966. doi: 10.1007/s10495-024-01976-z. Epub 2024 Jun 2. Apoptosis. 2024. PMID: 38824481 Free PMC article. Review. - Neurodegeneration Biomarkers in Adult Spinal Muscular Atrophy (SMA) Patients Treated with Nusinersen.
Andrés-Benito P, Vázquez-Costa JF, Ñungo Garzón NC, Colomina MJ, Marco C, González L, Terrafeta C, Domínguez R, Ferrer I, Povedano M. Andrés-Benito P, et al. Int J Mol Sci. 2024 Mar 29;25(7):3810. doi: 10.3390/ijms25073810. Int J Mol Sci. 2024. PMID: 38612621 Free PMC article. - Endothelial DR6 in blood-brain barrier malfunction in Alzheimer's disease.
Huang X, Qi J, Su Y, Zhou Y, Wang Q, Huang T, Xue D, Zeng Y, Verkhratsky A, Zhou B, Chen H, Yi C. Huang X, et al. Cell Death Dis. 2024 Apr 12;15(4):258. doi: 10.1038/s41419-024-06639-0. Cell Death Dis. 2024. PMID: 38609388 Free PMC article. - Preparation of Viable Human Neurites for Neurobiological and Neurodegeneration Studies.
Brüll M, Geese N, Celardo I, Laumann M, Leist M. Brüll M, et al. Cells. 2024 Jan 27;13(3):242. doi: 10.3390/cells13030242. Cells. 2024. PMID: 38334634 Free PMC article.
References
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–71. - PubMed
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–56. - PubMed
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. - PubMed
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials