A mechanosensitive transcriptional mechanism that controls angiogenesis - PubMed (original) (raw)
A mechanosensitive transcriptional mechanism that controls angiogenesis
Akiko Mammoto et al. Nature. 2009.
Abstract
Angiogenesis is controlled by physical interactions between cells and extracellular matrix as well as soluble angiogenic factors, such as VEGF. However, the mechanism by which mechanical signals integrate with other microenvironmental cues to regulate neovascularization remains unknown. Here we show that the Rho inhibitor, p190RhoGAP (also known as GRLF1), controls capillary network formation in vitro in human microvascular endothelial cells and retinal angiogenesis in vivo by modulating the balance of activities between two antagonistic transcription factors, TFII-I (also known as GTF2I) and GATA2, that govern gene expression of the VEGF receptor VEGFR2 (also known as KDR). Moreover, this new angiogenesis signalling pathway is sensitive to extracellular matrix elasticity as well as soluble VEGF. This is, to our knowledge, the first known functional cross-antagonism between transcription factors that controls tissue morphogenesis, and that responds to both mechanical and chemical cues.
Figures
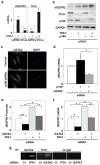
Fig. 1. TFII-I and GATA2 control VEGFR2 expression via p190RhoGAP
a) VEGFR2 and TFII-I mRNA levels in cells treated with TFII-I siRNA or lentiviral vectors (virus) relative to control cells (*, p < 0.05; unpaired Student's t-test is used throughout). b) Immunoblots showing VEGFR2, TFII-I, p190RhoGAP, and GAPDH protein levels in cells treated with TFII-I or p190RhoGAP siRNAs, or both. c) Immunofluorescence micrographs showing GATA2 distribution and nuclear DAPI staining in control and p190RhoGAP knockdown cells cultured with 0.3% serum (bar, 5μm). d) VEGFR2 mRNA level in cells transfected with p190RhoGAP siRNA alone or with GATA2 siRNA (*, p<0.01). VEGFR2 promoter activities (e) and mRNA levels (f) in cells transfected with GATA2 or TFII-I siRNA alone or in combination (*, p<0.01; **, p<0.05). g) ChIP analysis showing VEGFR2 promoter co-immunoprecipitating with GATA2 or TFII-I antibodies in cells transfected with TFII-I or GATA2 siRNA (Ct, control; n=3; error bars = s.e.m. throughout).
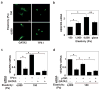
Fig. 2. Matrix elasticity controls VEGFR2 expression via TFII-I and GATA2
a) Immunofluorescence micrographs showing GATA2 and TFII-I distribution in HMVE cells cultured on the fibronectin-coated gels of different stiffness (Young's moduli of 150 and 4000 Pa) in EBM2 with 0.3% serum (bar, 5μm). b) VEGFR2 mRNA level in HMVE cells cultured on rigid fibronectin-coated glass or the gels of different elasticity (150, 1000 and 4000 Pa; normalized to that in cells on the softest gels; *, p<0.01, **, p<0.05). c) VEGFR2 mRNA levels in HMVE cells on soft gels (150 Pa) transfected with TFII-I or GATA2 siRNA alone, or in combination (normalized to cells on the stiffest gels; *, p<0.01). d) VEGFR2 mRNA levels in HMVE cells on soft gels transfected with p190RhoGAP or GATA2 siRNA alone, or in combination (normalized to cells on stiffest gels; *, p<0.01). Error bars represent s.e.m. of 3 replica experiments.
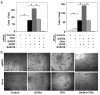
Fig. 3. Antagonism between GATA2 and TFII-I controls capillary cell migration and tube formation in vitro
a) The motility of HMVE cells transfected with human siRNAs or transduced with lentiviral vectors encoding GATA2 or TFII-I, alone or in combination, was quantitated using the Transwell migration assay (*, p< 0.01). Where indicated, VEGF (10 ng/ml) was added to the lower chamber and SU5416 was added in both chambers. Error bars represent s.e.m. of 3 replica experiments. b) Micrographs showing in vitro tube formation induced by VEGF (10 ng/ml) in HMVE cells transfected with siRNAs or transduced with lentiviral vectors encoding GATA2 or TFII-I, alone or in combination.
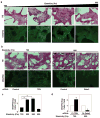
Fig. 4. Matrix elasticity controls vessel formation via TFII-I and GATA2 in vivo
Light (H&E-stained) (a,b top), immunofluorescence micrographs (a,b bottom) and vessel densities per high power field (c,d) showing cell infiltration and VEGFR2 expression in Matrigel with different elasticity implanted in mice for 7 days without (a,c) or with TFII-I or Gata2 siRNAs (b,d) (bar, 25 μm). The number of VEGFR2-positive blood vessels was normalized to that in the 700Pa gels in (c) and to that in gels treated with control siRNA in (d) (n=6, mean ± S.E.M., *, p< 0.01).
Fig. 5. TFII-I, GATA2, and p190RhoGAP regulate retinal vessel formation in vivo
a) Confocal fluorescence micrographs showing projected images of vessels stained with Alexa 594-isolectin in the retina of a control mouse eye versus eyes transfected with TFII-I, Gata2, or p190RhoGAP siRNAs (bar = 0.1 mm). b) Confocal fluorescence micrographs showing projected images of vessels stained with Alexa 594-isolectin in the retina of a control mouse eye versus eyes overexpressed TFII-I or GATA2, alone or both in combination (bar = 0.1 mm).
Similar articles
- VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling.
Heinolainen K, Karaman S, D'Amico G, Tammela T, Sormunen R, Eklund L, Alitalo K, Zarkada G. Heinolainen K, et al. Circ Res. 2017 Apr 28;120(9):1414-1425. doi: 10.1161/CIRCRESAHA.116.310477. Epub 2017 Mar 15. Circ Res. 2017. PMID: 28298294 Free PMC article. - Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1.
Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R. Takeda N, et al. Circ Res. 2004 Jul 23;95(2):146-53. doi: 10.1161/01.RES.0000134920.10128.b4. Epub 2004 Jun 10. Circ Res. 2004. PMID: 15192019 - Urokinase-type Plasminogen Activator (uPA) Promotes Angiogenesis by Attenuating Proline-rich Homeodomain Protein (PRH) Transcription Factor Activity and De-repressing Vascular Endothelial Growth Factor (VEGF) Receptor Expression.
Stepanova V, Jayaraman PS, Zaitsev SV, Lebedeva T, Bdeir K, Kershaw R, Holman KR, Parfyonova YV, Semina EV, Beloglazova IB, Tkachuk VA, Cines DB. Stepanova V, et al. J Biol Chem. 2016 Jul 15;291(29):15029-45. doi: 10.1074/jbc.M115.678490. Epub 2016 May 4. J Biol Chem. 2016. PMID: 27151212 Free PMC article. - Differential regulation of angiogenic cellular processes and claudin-5 by histamine and VEGF via PI3K-signaling, transcription factor SNAI2 and interleukin-8.
Laakkonen JP, Lappalainen JP, Theelen TL, Toivanen PI, Nieminen T, Jauhiainen S, Kaikkonen MU, Sluimer JC, Ylä-Herttuala S. Laakkonen JP, et al. Angiogenesis. 2017 Feb;20(1):109-124. doi: 10.1007/s10456-016-9532-7. Epub 2016 Nov 21. Angiogenesis. 2017. PMID: 27873103 - Transcription factors regulating vasculogenesis and angiogenesis.
Payne S, Neal A, De Val S. Payne S, et al. Dev Dyn. 2024 Jan;253(1):28-58. doi: 10.1002/dvdy.575. Epub 2023 Mar 6. Dev Dyn. 2024. PMID: 36795082 Free PMC article. Review.
Cited by
- Inhibition of angiogenesis and regenerative lung growth in Lepob/ob mice through adiponectin-VEGF/VEGFR2 signaling.
Hunyenyiwa T, Kyi P, Scheer M, Joshi M, Gasparri M, Mammoto T, Mammoto A. Hunyenyiwa T, et al. Front Cardiovasc Med. 2024 Oct 16;11:1491971. doi: 10.3389/fcvm.2024.1491971. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39479393 Free PMC article. - YAP/TAZ drives Notch and angiogenesis mechanoregulation in silico.
Passier M, Bentley K, Loerakker S, Ristori T. Passier M, et al. NPJ Syst Biol Appl. 2024 Oct 5;10(1):116. doi: 10.1038/s41540-024-00444-3. NPJ Syst Biol Appl. 2024. PMID: 39368976 Free PMC article. - The role of extracellular matrix in angiogenesis: Beyond adhesion and structure.
Libby JR, Royce H, Walker SR, Li L. Libby JR, et al. Biomater Biosyst. 2024 Jul 8;15:100097. doi: 10.1016/j.bbiosy.2024.100097. eCollection 2024 Sep. Biomater Biosyst. 2024. PMID: 39129826 Free PMC article. Review. - Utilization of a Cortical Xenogeneic Membrane for Guided Bone Regeneration: A Retrospective Case Series.
Debortoli C, Falguiere A, Campana F, Catherine JH, Tardivo D, Lan R. Debortoli C, et al. J Clin Med. 2024 Aug 5;13(15):4575. doi: 10.3390/jcm13154575. J Clin Med. 2024. PMID: 39124840 Free PMC article. - Biomimetic Marine-Sponge-Derived Spicule-Microparticle-Mediated Biomineralization and YAP/TAZ Pathway for Bone Regeneration In Vivo.
Choi S, Kim JH, Kang TH, An YH, Lee SJ, Hwang NS, Kim SH. Choi S, et al. Biomater Res. 2024 Jul 25;28:0056. doi: 10.34133/bmr.0056. eCollection 2024. Biomater Res. 2024. PMID: 39055902 Free PMC article.
References
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. - PubMed
- Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. - PubMed
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. - PubMed
- Dike LE, et al. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In vitro Cell Dev Biol Anim. 1999;35:441–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous