A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth - PubMed (original) (raw)
. 2009 Mar 15;69(6):2305-13.
doi: 10.1158/0008-5472.CAN-08-3795. Epub 2009 Feb 24.
Xi Yang, Feng Sun, Richeng Jiang, Douglas E Linn, Hege Chen, Hegang Chen, Xiangtian Kong, Jonathan Melamed, Clifford G Tepper, Hsing-Jien Kung, Angela M H Brodie, Joanne Edwards, Yun Qiu
Affiliations
- PMID: 19244107
- PMCID: PMC2672822
- DOI: 10.1158/0008-5472.CAN-08-3795
A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth
Zhiyong Guo et al. Cancer Res. 2009.
Abstract
The androgen receptor (AR) plays a key role in progression to incurable androgen ablation-resistant prostate cancer (PCA). We have identified three novel AR splice variants lacking the ligand-binding domain (designated as AR3, AR4, and AR5) in hormone-insensitive PCA cells. AR3, one of the major splice variants expressed in human prostate tissues, is constitutively active, and its transcriptional activity is not regulated by androgens or antiandrogens. Immunohistochemistry analysis on tissue microarrays containing 429 human prostate tissue samples shows that AR3 is significantly up-regulated during PCA progression and AR3 expression level is correlated with the risk of tumor recurrence after radical prostatectomy. Overexpression of AR3 confers ablation-independent growth of PCA cells, whereas specific knockdown of AR3 expression (without altering AR level) in hormone-resistant PCA cells attenuates their growth under androgen-depleted conditions in both cell culture and xenograft models, suggesting an indispensable role of AR3 in ablation-independent growth of PCA cells. Furthermore, AR3 may play a distinct, yet essential, role in ablation-independent growth through the regulation of a unique set of genes, including AKT1, which are not regulated by the prototype AR. Our data suggest that aberrant expression of AR splice variants may be a novel mechanism underlying ablation independence during PCA progression, and AR3 may serve as a prognostic marker to predict patient outcome in response to hormonal therapy. Given that these novel AR splice variants are not inhibited by currently available antiandrogen drugs, development of new drugs targeting these AR isoforms may potentially be effective for treatment of ablation-resistant PCA.
Figures
Figure 1
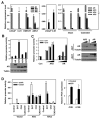
Cloning of novel alternative splice AR isoforms. (A) Cell lysates of different PCA cells were blotted with anti-AR(upper) and anti-Actin(lower). (B) CWR-R1 were infected with the lentivirus encoding the GFPshRNA(Control), ARshRNAa, ARshRNAb, and ARshRNAc(shARa, shARb and shARc) targeting different exons of AR as indicated in (D). At 48h-postinfection, cell lysates were subjected to western blot with anti-AR and anti-Actin, respectively. (C) Total RNA was isolated from CWR-R1 cells and reverse transcribed. The primer derived from AR shRNAc sequence or a control primer was used to perform 3′-RACE. (D) Schematic structure of the human AR splice variants. Hatched cassettes, cryptic exons; Solid thick lines, transcribed sequences.
Figure 2
Expression of AR isoforms in PCA cells. (A) The relative expression levels of AR, AR3, AR4 and AR5 were quantified using real-time PCR(left panel). The AR level in LNCaP was arbitrarily set as 1. AR3 expression in LNCaP and C-81 were further plotted with a higher resolution. *p<0.05(middle panel). Their expression in two pairs of CW22R xenograft tumors derived from the intact and castrated male mice were also quantified(right panel). *p<0.05. (B) Transcriptional activity of AR isoforms. COS-1 were transfected with ARR2-luciferase reporter together with the indicated expression vector. At 24h-posttransfection, the luciferase activity was measured. Cell lysates were blotted with anti-AR and anti-Tubulin, respectively (bottom). (C) COS-1 were transfected with ARR2-Luciferase reporter along with increasing doses of AR3 or AR expressing vector. At 24h-posttransfection, cells were treated with or without 10nM DHT for 24h and luciferase activities were measured. Cell lysates were blotted with anti-AR and anti-Tubulin, respectively (right panel). (D) LNCaP were infected with (+) or without(−) the lentivirus encoding ARshRNA (shAR) as described previously (26). At 6h-postinfection, cells were transfected with ARR2-Luciferase reporter along with AR3 or the codon-switched wild-type AR(ARcs). At 24h-posttransfection, cells were treated with DHT and casodex(CAS) as indicated for 24h before luciferase activity was measured (left panel). Cell lysates were blotted with anti-AR and anti-Tubulin (Suppl. Fig. 15). Right panel, LNCaP were transfected with AR3 vector or control. At 24h-posttransfection, cells were treated with or without Casodex (+CAS or −CAS), the relative PSA levels were quantified using real-time PCR. The PSA level in the control LNCaP was arbitrarily set as 1. *p<0.05.
Figure 3
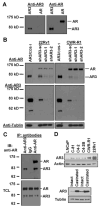
Detection of AR3 in hormone-insensitive PCA cells. (A) COS-1 were transfected with AR3 or AR vector. Total protein lysates were immunoblotted with anti-AR3 and anti-AR, respectively. (B) CWR-R1 and 22Rv1 were infected with lentivirus encoding AR3shRNAs (shAR3-1, −2) or the scrambled control (shAR3-sc). At 48h-postinfection, cell lysates were subjected to immunoblotting with anti-AR and anti-AR3, respectively. COS-1 overexpressing AR3 was used as a positive control (first lane). (C) CWR-R1 lysates were split into three equal aliquots and immnuoprecipitated with anti-AR3, control IgG and anti-AR, respectively. The resultant immunoprecipitates and the input total cell lysates (TCL) were immunoblotted with anti-AR. (D) Total cell lysates of a panel of PCA cells were blotted with anti-AR3 and anti-Actin, respectively (top panel). Bottom panel, extracts of two pairs of CW22R tumor xenografts derived from the intact and castrated male mice were blotted with anti-AR3 and anti-Tubulin, respectively.
Figure 4
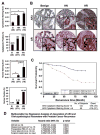
Increased AR3 expression in androgen-depletion-resistant PCA cells. (A) Human prostate tissue microarrays(TMAs) were stained with anti-AR3. The mean score of cytoplasmic and nuclear staining of the luminal cell as well as the positive rates for cytoplasmic and nuclear staining were shown. (Error bars indicate standard error, *p<0.01). Benign (B), hormone-naïve (HN) and hormone-resistant (HR). (B) The representative fields of TMAs stained with anti-AR3. The anti-AR stained arrays were included as a control. (C) Correlation of AR3 cytoplasmic staining with PSA recurrence after prostatectomy. (D) Multivariable Cox Regression Analysis.
Figure 5
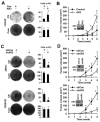
AR3 promotes PCA cell growth under androgen-depleted conditions. LNCaP were infected with lentivirus encoding AR3 or the control vector. After 2-week culture in the androgen-depleted (CS) or the complete(Com) medium, cells were visualized by Coomassie Blue staining. Under the same experimental conditions, cell numbers were quantified and plotted as a bargraph (right panel) (*p<0.05) (A). At 48h-postinfection, cells were injected into the castrated SCID mice and tumor growth were monitored weekly. The result represents the mean tumor volume ±SE (n = 5 mice/group), *p<0.05. Inset, Western blots of anti-AR3 and anti-AR of LNCaP xenograft tumor lysates (B). CWR-R1 and 22Rv1 cells were infected with lentivirus encoding AR3shRNA-1 (shAR3) or control shRNA (shCon). Cell growth was visualized and quantified as in Fig 5A (C). Tumor growth was monitored as in Fig 5B (D). Inset, Western blots of anti-AR3 and anti-AR of the CWR-R1 and 22Rv1 xenograft tumor lysates.
Figure 6
AKT1 is a target gene regulated by AR3. (A) Schematic representation of AR3 and AR target genes. (B) The effects of AR3 on AKT1 transcription. CWR-R1 and 22Rv1 were infected with lentivirus encoding the control shRNA(shCon), AR3shRNAs(shAR3-1 or shAR3-2), ARshRNA(shAR) or the scrambled control(shAR3-sc). At 48h-postinfection, the relative expression levels of AKT1 transcripts compared to the shCon was quantified by real-time PCR (*p<0.05) (Left Panel). The protein levels of AKT1, Actin, AR3, AR, pGSK3β and GSK3β were also detected by immunoblotting. The levels of AKT1 from the immunoblots were normalized by calculating the ratios of AKT1/actin. The changes in fold compared to the control were shown (bottom). Right Panels, The lysates of LNCaP and 22Rv1 xenograft tumors from Figure 5A and 5B were subjected to immunoblotting as described above. (C) The effects of AKT1 knockdown on PCA cell growth. CWR-R1 cells were infected with the lentivirus encoding two independent AKT1 shRNAs (shAKT1a and shAKT1b) respectively. After 2-week culture in androgen-depleted medium, cells were visualized and quantified as described in Fig 5A. (D) CWR-R1 and 22Rv1 were treated with or without DHT(10nM) for 1h. Binding of AR3 or AR to the putative ARE sites (P1, P2 and P3) of human AKT1 gene was analyzed by ChIP assays. The ARE at the PSA enhancer region (PSA-E) was used as a positive control for AR. PCR products from input(1), immunoprecipitation with anti-AR3(2), anti-AR(3) or the control antibody(4), were resolved on agarose gels(Left panel). The PCR products were quantified by using the software Quantity One(Right panel).
Similar articles
- ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors.
Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. Yamashita S, et al. Neoplasia. 2012 Jan;14(1):74-83. doi: 10.1593/neo.111436. Neoplasia. 2012. PMID: 22355276 Free PMC article. - Vav3 enhances androgen receptor splice variant activity and is critical for castration-resistant prostate cancer growth and survival.
Peacock SO, Fahrenholtz CD, Burnstein KL. Peacock SO, et al. Mol Endocrinol. 2012 Dec;26(12):1967-79. doi: 10.1210/me.2012-1165. Epub 2012 Sep 28. Mol Endocrinol. 2012. PMID: 23023561 Free PMC article. - Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes.
Kong D, Sethi S, Li Y, Chen W, Sakr WA, Heath E, Sarkar FH. Kong D, et al. Prostate. 2015 Feb;75(2):161-74. doi: 10.1002/pros.22901. Epub 2014 Oct 13. Prostate. 2015. PMID: 25307492 Free PMC article. - Androgen receptor action in hormone-dependent and recurrent prostate cancer.
Agoulnik IU, Weigel NL. Agoulnik IU, et al. J Cell Biochem. 2006 Oct 1;99(2):362-72. doi: 10.1002/jcb.20811. J Cell Biochem. 2006. PMID: 16619264 Review. - Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways.
Vis AN, Schröder FH. Vis AN, et al. BJU Int. 2009 Aug;104(4):438-48. doi: 10.1111/j.1464-410X.2009.08695.x. Epub 2009 Jun 24. BJU Int. 2009. PMID: 19558559 Review.
Cited by
- ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors.
Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. Yamashita S, et al. Neoplasia. 2012 Jan;14(1):74-83. doi: 10.1593/neo.111436. Neoplasia. 2012. PMID: 22355276 Free PMC article. - Role of androgen receptor splice variant-7 (AR-V7) in prostate cancer resistance to 2nd-generation androgen receptor signaling inhibitors.
Zhu Y, Dalrymple SL, Coleman I, Zheng SL, Xu J, Hooper JE, Antonarakis ES, De Marzo AM, Meeker AK, Nelson PS, Isaacs WB, Denmeade SR, Luo J, Brennen WN, Isaacs JT. Zhu Y, et al. Oncogene. 2020 Nov;39(45):6935-6949. doi: 10.1038/s41388-020-01479-6. Epub 2020 Sep 28. Oncogene. 2020. PMID: 32989253 Free PMC article. - Experimental treatment efficacy of dmrFABP5 on prostate cancer singly or in combination with drugs in use.
Abdulsamad SA, Naeem AA, Zeng H, He G, Jin X, Alenezi BA, Ai J, Zhang J, Ma H, Rudland PS, Ke Y. Abdulsamad SA, et al. Am J Cancer Res. 2024 Jan 15;14(1):300-323. doi: 10.62347/YPPT5752. eCollection 2024. Am J Cancer Res. 2024. PMID: 38323289 Free PMC article. - Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines.
Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Li Y, et al. Cancer Res. 2013 Jan 15;73(2):483-9. doi: 10.1158/0008-5472.CAN-12-3630. Epub 2012 Nov 1. Cancer Res. 2013. PMID: 23117885 Free PMC article. - Are androgen receptor variants a substitute for the full-length receptor?
Lu J, Van der Steen T, Tindall DJ. Lu J, et al. Nat Rev Urol. 2015 Mar;12(3):137-44. doi: 10.1038/nrurol.2015.13. Epub 2015 Feb 10. Nat Rev Urol. 2015. PMID: 25666893 Review.
References
- Litvinov IV, Antony L, Isaacs JT. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate. 2004;61:299–304. - PubMed
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. - PubMed
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. - PubMed
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA106504/CA/NCI NIH HHS/United States
- U01 CA086772/CA/NCI NIH HHS/United States
- R01 CA106504-04/CA/NCI NIH HHS/United States
- UO1-CA86772/CA/NCI NIH HHS/United States
- CA106504/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous