Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury - PubMed (original) (raw)
. 2009 Feb 25;29(8):2588-96.
doi: 10.1523/JNEUROSCI.5832-08.2009.
Ylva Carlsson, Emy Basso, Changlian Zhu, Catherine I Rousset, Andrea Rasola, Bengt R Johansson, Klas Blomgren, Carina Mallard, Paolo Bernardi, Michael A Forte, Henrik Hagberg
Affiliations
- PMID: 19244535
- PMCID: PMC3049447
- DOI: 10.1523/JNEUROSCI.5832-08.2009
Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury
Xiaoyang Wang et al. J Neurosci. 2009.
Abstract
Cyclophilin D (CypD), a regulator of the mitochondrial membrane permeability transition pore (PTP), enhances Ca(2+)-induced mitochondrial permeabilization and cell death in the brain. However, the role of CypD in hypoxic-ischemic (HI) brain injury at different developmental ages is unknown. At postnatal day (P) 9 or P60, littermates of CypD-deficient [knock-out (KO)], wild-type (WT), and heterozygous mice were subjected to HI, and brain injury was evaluated 7 d after HI. CypD deficiency resulted in a significant reduction of HI brain injury at P60 but worsened injury at P9. After HI, caspase-dependent and -independent cell death pathways were more induced in P9 CypD KO mice than in WT controls, and apoptotic activation was minimal at P60. The PTP had a considerably higher induction threshold and lower sensitivity to cyclosporin A in neonatal versus adult mice. On the contrary, Bax inhibition markedly reduced caspase activation and brain injury in immature mice but was ineffective in the adult brain. Our findings suggest that CypD/PTP is critical for the development of brain injury in the adult, whereas Bax-dependent mechanisms prevail in the immature brain. The role of CypD in HI shifts from a predominantly prosurvival protein in the immature to a cell death mediator in the adult brain.
Figures
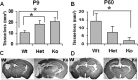
Figure 1.
Effect of CypD deficiency on HI injury in neonatal (P9) and adult (P60) mice. Volume of brain total tissue loss in P9 (A) and P60 (B) mice. Representative photomicrographs of MAP2-stained sections of P9 (C) and P60 (D) mouse brains. P9 mice: WT, n = 16; Het, n = 40; KO, n = 19. P60 mice: WT, n = 18; Het, n = 28; KO, n = 13. *p < 0.05.
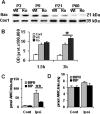
Figure 2.
Brain mitochondria morphology and PTP-relevant molecular expression in WT and CypD KO neonatal (P9) versus adult (P60) mice. A, Electron microscopy; morphology of isolated control brain mitochondria in WT versus KO mice at P9 and P60. n = 2 per group. Scale bar, 200 nm. B–E, Representative immunoblotting pictures (B, D) and immunoblotting quantification (C, E) of mitochondrial CypD (CyD), and ANT expression in WT and KO mice at P3, P9, P21, and P60 (B, C), as well as 6 and 24 h after HI in the ipsilateral hemispheres (D, E). n = 6 per group. The mitochondrial marker Cox1 was used as protein loading control. Cont, Normal control brains from WT mice; OD, optical density.
Figure 3.
CRC of WT and CypD KO mouse brain mitochondria in neonatal P9 and adult P60 mice. Effect of CsA. A, The incubation medium contained 120 m
m
KCl, 10 m
m
Tris–3-(_N_-morpholino)-propanesulfonic acid, 5 m
m
glutamate-Tris, 2.5 m
m
malate-Tris, 1 m
m
Pi-Tris, 20 μ
m
EGTA-Tris, and 1 μ
m
Calcium Green-5N, pH 7.4. The experiments were started by the addition of 1 mg of mitochondria from WT (traces d–g) or KO (traces d′–g′) mice, followed 1 min later by the indicated concentrations of Ca2+ (arrows); in the experiments of traces e, e′, g, and g′, 0.8 μ
m
CsA was added before adding mitochondria. Traces shown are representative of at least four experiments. B, Quantitative assessment of the mitochondrial CRC. Error bars refer to SEM of at least four replicate experiments per condition. C, Ratio between the CRC detected in the presence (CRC) and absence (CRC0) of 0.8 μ
m
CsA. **p < 0.01, compared between neonatal WT and adult WT mice; #p < 0.05, compared between WT and KO of the same age.
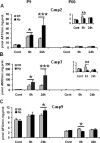
Figure 4.
Effect of CypD deficiency on apoptosis after HI in neonatal (P9) and adult (P60) mice. Caspase-2 (A), caspase-3 (B), and caspase-9 (C) activation at 6 and 24 h after HI in P9 (left column) and P60 (right column) mice in ipsilateral hemispheres. Cont, Normal control brains without any treatment. *p < 0.05, **p < 0.01, ***p < 0.001, compared between WT and KO at the same time point.
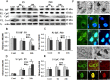
Figure 5.
Effect of CypD deficiency on mitochondrial proapoptotic protein release after HI in neonatal (P9) and adult (P60) mice. A, Representative immunoblotting pictures show AIF and cyt c release from mitochondria 6 and 24 h after HI in ipsilateral hemispheres. 6h, 24h, Six or 24 h after HI; Cont, normal control brains from WT mice. B, C, Quantification of immunoblotting for AIF in the mitochondria-enriched cell fractions (P2) at P9 and P60. D, E, Quantification of immunoblotting for cyt c in the cytosolic cell fractions (S) at P9 and P60. *p < 0.05. F, Representative pictures of AIF and cyt c immunostaining in CypD KO mouse brains at P9. AIF in a normal control brain (a, c, d) and 24 h after HI (b, f, g). cyt c in a normal control brain (i, k, l) and 24 h after HI (j, n, o). Note the more condensed apoptotic phenotype of the nuclei in h. c, f, AIF staining; e, h, DAPI nuclei staining; d, g, merged pictures of AIF (green) and DAPI (blue); k, n, cyt c staining; m, p, caspase-3 staining; l, o, merged pictures of cyt c (green) and caspase-3 (red). Scale bars, 10 μm.
Figure 6.
Bax expression during development and after HI in WT and CypD KO mice brain mitochondria in neonatal (P9) and adult (P60) mice. A, Representative immunoblotting pictures of Bax expression at P3, P9, P21, and P60. B, Quantification of immunoblotting for Bax in mitochondria-enriched cell fractions after HI at P9. Cont, Normal control brain from P9 WT mice. C, D, Effect of Bax inhibition in neonatal and adult brain injury in WT mice. Caspase-3 activation 24 h after HI in P9 (C) and P60 (D) WT mouse brains. Cont, Normal control brains from WT mice; Ipsi, ipsilateral hemisphere. *p < 0.05; **p < 0.01.
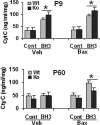
Figure 7.
BH3 peptide induced the release of cyt c from isolated brain mitochondria of WT and CypD KO mice in neonates (P9, top) and adults (P60, bottom), measured by ELISA. Cont, Control peptide; Veh, vehicle control for Bax [50 m
m
Tris-HCl and 10 m
m
reduced glutathione (L-form), pH 8.0]; Bax, full-length recombinant protein of Bax (100 n
m
). *p < 0.05.
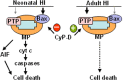
Figure 8.
MP is center stage in HI brain injury at all ages, but the mechanism of MP shifts during brain development. In neonatal mice, Bax-mediated permeabilization leading to AIF- and caspase-dependent cell death, which is inhibited by CypD, predominates. In contrast, in adult animals, cell death depends on CypD-regulated and PTP-mediated mitochondrial permeabilization.
Similar articles
- Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia.
Svedin P, Hagberg H, Sävman K, Zhu C, Mallard C. Svedin P, et al. J Neurosci. 2007 Feb 14;27(7):1511-8. doi: 10.1523/JNEUROSCI.4391-06.2007. J Neurosci. 2007. PMID: 17301159 Free PMC article. - Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice.
King AL, Swain TM, Mao Z, Udoh US, Oliva CR, Betancourt AM, Griguer CE, Crowe DR, Lesort M, Bailey SM. King AL, et al. Am J Physiol Gastrointest Liver Physiol. 2014 Feb 15;306(4):G265-77. doi: 10.1152/ajpgi.00278.2013. Epub 2013 Dec 19. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24356880 Free PMC article. - Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia.
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Schinzel AC, et al. Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12005-10. doi: 10.1073/pnas.0505294102. Epub 2005 Aug 15. Proc Natl Acad Sci U S A. 2005. PMID: 16103352 Free PMC article. - Apoptotic mechanisms in the immature brain: involvement of mitochondria.
Hagberg H, Mallard C, Rousset CI, Xiaoyang Wang. Hagberg H, et al. J Child Neurol. 2009 Sep;24(9):1141-6. doi: 10.1177/0883073809338212. Epub 2009 Jul 2. J Child Neurol. 2009. PMID: 19574577 Free PMC article. Review. - Cyclophilin D: An Integrator of Mitochondrial Function.
Amanakis G, Murphy E. Amanakis G, et al. Front Physiol. 2020 Jun 17;11:595. doi: 10.3389/fphys.2020.00595. eCollection 2020. Front Physiol. 2020. PMID: 32625108 Free PMC article. Review.
Cited by
- Tamoxifen-induced knockdown of the mitochondrial calcium uniporter in Thy1-expressing neurons protects mice from hypoxic/ischemic brain injury.
Nichols M, Pavlov EV, Robertson GS. Nichols M, et al. Cell Death Dis. 2018 May 22;9(6):606. doi: 10.1038/s41419-018-0607-9. Cell Death Dis. 2018. PMID: 29789575 Free PMC article. - Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis.
Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Novgorodov SA, et al. J Biol Chem. 2011 Feb 11;286(6):4644-58. doi: 10.1074/jbc.M110.164392. Epub 2010 Dec 10. J Biol Chem. 2011. PMID: 21148554 Free PMC article. - Plasticity in the Neonatal Brain following Hypoxic-Ischaemic Injury.
Rocha-Ferreira E, Hristova M. Rocha-Ferreira E, et al. Neural Plast. 2016;2016:4901014. doi: 10.1155/2016/4901014. Epub 2016 Mar 7. Neural Plast. 2016. PMID: 27047695 Free PMC article. Review. - The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology.
Bernardi P, Rasola A, Forte M, Lippe G. Bernardi P, et al. Physiol Rev. 2015 Oct;95(4):1111-55. doi: 10.1152/physrev.00001.2015. Physiol Rev. 2015. PMID: 26269524 Free PMC article. Review. - Cyclophilin D in mitochondrial pathophysiology.
Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Giorgio V, et al. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1113-8. doi: 10.1016/j.bbabio.2009.12.006. Epub 2009 Dec 21. Biochim Biophys Acta. 2010. PMID: 20026006 Free PMC article. Review.
References
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. - PubMed
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. - PubMed
- Cheng Y, Gidday JM, Yan Q, Shah AR, Holtzman DM. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. - PubMed
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0802853/MRC_/Medical Research Council/United Kingdom
- R01 GM069883/GM/NIGMS NIH HHS/United States
- R01 GM069883-06/GM/NIGMS NIH HHS/United States
- GM69883/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous