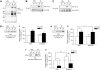The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis - PubMed (original) (raw)
. 2009 May 14;113(20):4942-54.
doi: 10.1182/blood-2008-08-174318. Epub 2009 Feb 25.
Michael G Tomlinson, Stuart Ellison, Alexandra Mazharian, Jenson Lim, Yan Zhao, Kristin N Kornerup, Jocelyn M Auger, Steve G Thomas, Tarvinder Dhanjal, Neena Kalia, Jing W Zhu, Arthur Weiss, Steve P Watson
Affiliations
- PMID: 19246339
- PMCID: PMC2686144
- DOI: 10.1182/blood-2008-08-174318
The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis
Yotis A Senis et al. Blood. 2009.
Abstract
Platelets play a fundamental role in hemostasis and thrombosis. They are also involved in pathologic conditions resulting from blocked blood vessels, including myocardial infarction and ischemic stroke. Platelet adhesion, activation, and aggregation at sites of vascular injury are regulated by a diverse repertoire of tyrosine kinase-linked and G protein-coupled receptors. Src family kinases (SFKs) play a central role in initiating and propagating signaling from several platelet surface receptors; however, the underlying mechanism of how SFK activity is regulated in platelets remains unclear. CD148 is the only receptor-like protein tyrosine phosphatase identified in platelets to date. In the present study, we show that mutant mice lacking CD148 exhibited a bleeding tendency and defective arterial thrombosis. Basal SFK activity was found to be markedly reduced in CD148-deficient platelets, resulting in a global hyporesponsiveness to agonists that signal through SFKs, including collagen and fibrinogen. G protein-coupled receptor responses to thrombin and other agonists were also marginally reduced. These results highlight CD148 as a global regulator of platelet activation and a novel antithrombotic drug target.
Figures
Figure 1
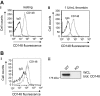
CD148 is expressed in human and mouse platelets. (A) Human platelets. (Ai) Resting and (Aii) thrombin-activated (1 U/mL) human platelets were stained with a mouse anti–human CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of CD148, or the same amount of an isotype control antibody (IgG, black line), followed by a FITC-conjugated anti–mouse IgG secondary antibody and analyzed by flow cytometry. No detectable change in CD148 surface expression was observed in thrombin-activated platelets. (B) Mouse platelets. (Bi) Resting wild-type (WT) mouse platelets were incubated with either hamster anti–mouse CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of mouse CD148, or an isotype control antibody (IgG, black line). Platelets were subsequently stained with FITC-conjugated anti–hamster secondary antibody and analyzed by flow cytometry. (Bii) Whole-cell lysates (WCLs) prepared of WT and CD148 transmembrane-knockout (KO) mouse platelets were Western blotted for CD148. A 220-kDa band was detected in the WT sample but not in the KO sample.
Figure 2
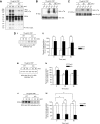
CD148-deficient platelets exhibit impaired GPVI-mediated platelet aggregation, secretion, and integrin activation. (A) Washed platelets (2 × 108/mL) prepared from wild-type (WT), CD148 transmembrane-knockout (CD148 TM-KO), and FcR γ-chain heterozygous-deficient (γ-chain+/−) mice were stimulated with low, intermediate, and high doses of: (Ai) collagen-related peptide (CRP; 1, 3, and 10 μg/mL); (Aii) collagen (1, 3, and 10 μg/mL); and (Aiii) thrombin (0.03, 0.09, and 0.3 U/mL). Platelet-rich plasma prepared from WT and CD148 TM-KO mice was stimulated with low, intermediate, and high doses of (Aiv) thromboxane A2 analog U46619 (1, 3, and 10 μM) and (Av) ADP (1, 3, and 10 μM). Platelet aggregation was measured as a change in light transmission, and ATP secretion was measured as luciferin/luciferase-mediated luminescence, using a lumi-aggregometer. Representative images are shown (n = 3-6 mice per condition). (B) Surface expression of P-selectin and (C) “active” integrin αIIbβ3 was quantified on resting and activated (10 μg/mL CRP or 0.06 U/mL thrombin) platelets from litter-matched WT and CD148 TM-KO (KO) mice by flow cytometry. Resting (black lines) and activated (gray lines) platelets were stained with either (Bi) FITC-conjugated rat anti–mouse P-selectin antibody or (Ci) PE-conjugated JON/A antibody to “active” integrin αIIbβ3. Data presented as (Bii) P-selectin geometric mean fluorescence intensity (MFI) and (Cii) fold increase in JON/A binding relative to total αIIbβ3 staining. Representative histograms are shown (n = 3 mice per condition). Bar height and error bars represent mean ± SEM (*P < .05, **P < .01).
Figure 2
CD148-deficient platelets exhibit impaired GPVI-mediated platelet aggregation, secretion, and integrin activation. (A) Washed platelets (2 × 108/mL) prepared from wild-type (WT), CD148 transmembrane-knockout (CD148 TM-KO), and FcR γ-chain heterozygous-deficient (γ-chain+/−) mice were stimulated with low, intermediate, and high doses of: (Ai) collagen-related peptide (CRP; 1, 3, and 10 μg/mL); (Aii) collagen (1, 3, and 10 μg/mL); and (Aiii) thrombin (0.03, 0.09, and 0.3 U/mL). Platelet-rich plasma prepared from WT and CD148 TM-KO mice was stimulated with low, intermediate, and high doses of (Aiv) thromboxane A2 analog U46619 (1, 3, and 10 μM) and (Av) ADP (1, 3, and 10 μM). Platelet aggregation was measured as a change in light transmission, and ATP secretion was measured as luciferin/luciferase-mediated luminescence, using a lumi-aggregometer. Representative images are shown (n = 3-6 mice per condition). (B) Surface expression of P-selectin and (C) “active” integrin αIIbβ3 was quantified on resting and activated (10 μg/mL CRP or 0.06 U/mL thrombin) platelets from litter-matched WT and CD148 TM-KO (KO) mice by flow cytometry. Resting (black lines) and activated (gray lines) platelets were stained with either (Bi) FITC-conjugated rat anti–mouse P-selectin antibody or (Ci) PE-conjugated JON/A antibody to “active” integrin αIIbβ3. Data presented as (Bii) P-selectin geometric mean fluorescence intensity (MFI) and (Cii) fold increase in JON/A binding relative to total αIIbβ3 staining. Representative histograms are shown (n = 3 mice per condition). Bar height and error bars represent mean ± SEM (*P < .05, **P < .01).
Figure 3
GPVI proximal signaling is defective in CD148-deficient platelets. (A) Whole-cell lysates (WCLs) prepared of resting and collagen-related peptide (CRP)–activated platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were Western blotted with an anti-phosphotyrosine antibody (p-Tyr). Platelets were stimulated with 10 μg/mL CRP for 90 and 300 seconds (sec). Bands corresponding to Src family kinases (SFKs) and FcR γ-chain are indicated. (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with a anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs prepared of platelets stimulated for 0, 30, and 90 seconds with CRP were Western blotted for (Di) Lyn p-Tyr-507, (Diii) Src p-Tyr-529, and (Dv) SFK activation loop p-Tyr. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
Figure 4
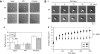
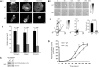
CD148 is essential for αIIbβ3-mediated platelet spreading. (Ai) Washed platelets (2 × 107/mL) from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were treated with 10 μM indomethacin and 2 U/mL apyrase in the absence (basal) and presence of either 10 μM PP1 or 1 U/mL thrombin before being placed on a fibrinogen-coated surface for 45 minutes. Platelets were fixed and images captured by differential interference contrast (DIC) microscopy (5 μm scale bar). (Aii) Surface areas of platelets per condition shown in (Ai) were measured using ImageJ software (mean ± SEM; n = 250-500 individual platelets per condition). (Bi) Representative DIC images of WT and KO platelets captured in real time at 0, 5, 10, 15, and 20 minutes (min) after being placed on a fibrinogen-coated surface, in the presence of 10 μM indomethacin and 2 U/mL apyrase. (Bii) Surface areas of individual platelets were measured at various time points during spreading on fibrinogen using ImageJ software (mean ± SEM; n = 6-10 platelets per time point). P value was calculated by 2-way analysis of variance. Results are representative of 3 WT and 3 KO mice. See also Figure S4 Videos S1,S2.
Figure 5
Defective αIIbβ3 signaling in CD148-deficient platelets. Platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on BSA- and fibrinogen-coated surfaces for 45 minutes. Whole cell lysates (WCLs) were prepared of BSA (BSA) nonadherent and fibrinogen (fib) adherent platelets. (A) Equal amounts of total protein were resolved by SDS-PAGE and Western blotted with an anti-phosphotyrosine antibody (p-Tyr). (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with an anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs were Western blotted with (Di) an anti-Src p-Tyr-529 antibody, (Diii) an anti-Lyn p-Tyr-507 antibody, and (Dv) an anti-Src family kinase (SFK) activation loop p-Tyr antibody. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
Figure 6
CD148-deficient megakaryocytes do not spread or migrate. (Ai) Cultured bone marrow–derived megakaryocytes from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on fibrinogen-, collagen-, and fibronectin-coated surfaces. Adherent megakaryocytes were fixed, permeablized, and actin fibers stained with rhodamine-phalloidin. Representative images from 3 separate experiments (20 μm scale bar). (Aii) The surface area of spread megakaryocytes was quantified and presented as mean ± SEM. (Bi) WT and KO megakaryocytes were placed on a fibronectin-coated surface and allowed to migrate toward a SDF-1α gradient in a Dunn chamber. Images were captured at the indicated times by differential interference contrast microscopy (20 μm scale bar). (Bii) The direction and distance traveled by individual megakaryocytes was tracked in real time and plotted as shown. Each trace was generated by a single megakaryocyte over a 4-hour period. Representative images from 3 separate experiments. See also Figure S6 Videos S3,S4. (C) Whole-cell lysates (WCLs) prepared of nonadherent WT, and KO megakaryocytes were Western blotted with an anti-Src family kinase (SFK) activation p-Tyr antibody. Membranes were stripped and reblotted with an anti–Src-pan antibody (Src). Representative images from 2 experiments. (D) Delayed thrombopoiesis in CD48 TM-KO mice after complement-mediated immune thrombocytopenia. Thrombocytopenia was induced in WT and KO mice by an intraperitoneal injection of anti–mouse GPIbα antibody (2 μg/g of mouse). Peripheral platelet counts were measured 7 days before injection (time = 0) and at 3, 48, 72, 96, 120, 144, and 172 hours after injection (n = 3-4 mice per time point). Mean platelet count (± SEM) was plotted as a function of time (**P < .01).
Figure 7
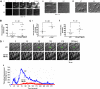
CD148 positively regulates platelet aggregate formation on collagen under flow and thrombus formation in vivo. (Ai) Anticoagulated blood from wild-type (WT) and CD148 transmembrane-knockout (KO) mice was flowed through collagen-coated capillary tubes at 1000 s−1. Platelets were fluorescently labeled with DiOC6 before being flowed. Representative images were taken in real time by fluorescence microscopy (10 μm scale bar). (Aii) Differential interference contrast (DIC) images of fixed platelets on collagen fibrils after being flowed through collagen-coated capillary tubes at 1000 s−1 for 4 minutes (10 μm scale bar). (Aiii) High magnification DIC images of adherent platelets from panel (Aii) (5 μm scale bar). Results are representative of 3 WT and 3 KO mice. See also Figure S7 Videos S5,S6. (B-D) The functional role of CD148 in thrombosis and hemostasis was investigated using 3 different in vivo assays: (B) the tail bleeding assay, (C) the ferric chloride injury model, and (D) the laser injury model. (B) CD148 transmembrane-knockout (CD148 TM-KO) mice exhibited prolonged bleeding compared with litter-matched WT mice, after excision of a small portion of the tail tip. Symbols represent individual mice. Horizontal lines intersecting datasets represent the mean. (Ci) FcR γ-chain–deficient (γ-chain−/−) and (Cii) CD148 TM-KO mice exhibited delayed vascular occlusion after ferric chloride–induced injury of mesenteric arterioles compared with litter-matched WT mice. (Di) Platelets from WT and CD148 TM-KO mice were fluorescently labeled ex vivo with rat anti–mouse αIIb primary antibody and Alex488-conjugated secondary antibody before being reintroduced into recipient mice. Arterioles in cremaster muscles of recipients were subsequently injured by laser, and the accumulation of platelets (green) into the thrombi was assessed. Representative images from 5 WT and 5 CD148 TM-KO mice are shown. (Dii) Each curve represents the median integrated thrombus fluorescence intensity in arbitrary units (a.u.) for 25 thrombi induced in 5 mice of each genotype. See also Figure S7 Videos S7,S8.
Similar articles
- CD148 enhances platelet responsiveness to collagen by maintaining a pool of active Src family kinases.
Ellison S, Mori J, Barr AJ, Senis YA. Ellison S, et al. J Thromb Haemost. 2010 Jul;8(7):1575-83. doi: 10.1111/j.1538-7836.2010.03865.x. Epub 2010 Mar 23. J Thromb Haemost. 2010. PMID: 20345711 - Maintenance of murine platelet homeostasis by the kinase Csk and phosphatase CD148.
Mori J, Nagy Z, Di Nunzio G, Smith CW, Geer MJ, Al Ghaithi R, van Geffen JP, Heising S, Boothman L, Tullemans BME, Correia JN, Tee L, Kuijpers MJE, Harrison P, Heemskerk JWM, Jarvis GE, Tarakhovsky A, Weiss A, Mazharian A, Senis YA. Mori J, et al. Blood. 2018 Mar 8;131(10):1122-1144. doi: 10.1182/blood-2017-02-768077. Epub 2018 Jan 4. Blood. 2018. PMID: 29301754 Free PMC article. - Dominant role of the protein-tyrosine phosphatase CD148 in regulating platelet activation relative to protein-tyrosine phosphatase-1B.
Mori J, Wang YJ, Ellison S, Heising S, Neel BG, Tremblay ML, Watson SP, Senis YA. Mori J, et al. Arterioscler Thromb Vasc Biol. 2012 Dec;32(12):2956-65. doi: 10.1161/ATVBAHA.112.300447. Epub 2012 Oct 11. Arterioscler Thromb Vasc Biol. 2012. PMID: 23065825 - Perspective: Tyrosine phosphatases as novel targets for antiplatelet therapy.
Tautz L, Senis YA, Oury C, Rahmouni S. Tautz L, et al. Bioorg Med Chem. 2015 Jun 15;23(12):2786-97. doi: 10.1016/j.bmc.2015.03.075. Epub 2015 Apr 4. Bioorg Med Chem. 2015. PMID: 25921264 Free PMC article. Review. - Platelet immunoreceptor tyrosine-based activation motif (ITAM) and hemITAM signaling and vascular integrity in inflammation and development.
Lee RH, Bergmeier W. Lee RH, et al. J Thromb Haemost. 2016 Apr;14(4):645-54. doi: 10.1111/jth.13250. Epub 2016 Feb 16. J Thromb Haemost. 2016. PMID: 26749528 Review.
Cited by
- LAIR-1 and PECAM-1 function via the same signaling pathway to inhibit GPVI-mediated platelet activation.
Smith CW, Nagy Z, Geer MJ, Pike JA, Patel P, Senis YA, Mazharian A. Smith CW, et al. Res Pract Thromb Haemost. 2024 Aug 23;8(6):102557. doi: 10.1016/j.rpth.2024.102557. eCollection 2024 Aug. Res Pract Thromb Haemost. 2024. PMID: 39318773 Free PMC article. - The protein tyrosine phosphatase DEP-1/PTPRJ promotes breast cancer cell invasion and metastasis.
Spring K, Fournier P, Lapointe L, Chabot C, Roussy J, Pommey S, Stagg J, Royal I. Spring K, et al. Oncogene. 2015 Oct 29;34(44):5536-47. doi: 10.1038/onc.2015.9. Epub 2015 Mar 16. Oncogene. 2015. PMID: 25772245 - Loss of the protein-tyrosine phosphatase DEP-1/PTPRJ drives meningioma cell motility.
Petermann A, Haase D, Wetzel A, Balavenkatraman KK, Tenev T, Gührs KH, Friedrich S, Nakamura M, Mawrin C, Böhmer FD. Petermann A, et al. Brain Pathol. 2011 Jul;21(4):405-18. doi: 10.1111/j.1750-3639.2010.00464.x. Epub 2010 Dec 27. Brain Pathol. 2011. PMID: 21091576 Free PMC article. - Interplay between the tyrosine kinases Chk and Csk and phosphatase PTPRJ is critical for regulating platelets in mice.
Nagy Z, Mori J, Ivanova VS, Mazharian A, Senis YA. Nagy Z, et al. Blood. 2020 Apr 30;135(18):1574-1587. doi: 10.1182/blood.2019002848. Blood. 2020. PMID: 32016283 Free PMC article. - The translation attenuating arginine-rich sequence in the extended signal peptide of the protein-tyrosine phosphatase PTPRJ/DEP1 is conserved in mammals.
Karagyozov L, Grozdanov PN, Böhmer FD. Karagyozov L, et al. PLoS One. 2020 Dec 9;15(12):e0240498. doi: 10.1371/journal.pone.0240498. eCollection 2020. PLoS One. 2020. PMID: 33296397 Free PMC article.
References
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin αIIbβ3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. - PubMed
- Woodside DG, Obergfell A, Talapatra A, Calderwood DA, Shattil SJ, Ginsberg MH. The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J Biol Chem. 2002;277:39401–39408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/08/034/25 085/BHF_/British Heart Foundation/United Kingdom
- 073107/WT_/Wellcome Trust/United Kingdom
- FS/08/062/25797/BHF_/British Heart Foundation/United Kingdom
- CH/03/003/BHF_/British Heart Foundation/United Kingdom
- 65 282/MRC_/Medical Research Council/United Kingdom
- PG/07/034/22 775/BHF_/British Heart Foundation/United Kingdom
- FS/08/034/25085/BHF_/British Heart Foundation/United Kingdom
- AI066120/AI/NIAID NIH HHS/United States
- FS/05/085/19 460/BHF_/British Heart Foundation/United Kingdom
- FS/08/062/25 797/BHF_/British Heart Foundation/United Kingdom
- R01 AI066120/AI/NIAID NIH HHS/United States
- 10 717/MRC_/Medical Research Council/United Kingdom
- G0400247/MRC_/Medical Research Council/United Kingdom
- G0300102/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous