New roles for endosomes: from vesicular carriers to multi-purpose platforms - PubMed (original) (raw)
Review
New roles for endosomes: from vesicular carriers to multi-purpose platforms
Gwyn W Gould et al. Nat Rev Mol Cell Biol. 2009 Apr.
Abstract
The careful sorting and recycling of membranes and cargo and the intracellular delivery of proteins, toxins and viruses by endocytosis are well-established roles for the endocytic apparatus, which is present in all eukaryotic cells. Recently, it has become clear that endosomes have key roles in such diverse processes as cytokinesis, polarization and migration, in which their functions might be distinct from those classically associated with endosomes. We speculate that endosomes function as multifunctional platforms on which unique sets of molecular machines are assembled to suit different cellular roles.
Figures
Figure 1. Endosome platforms in cytokinesis
For clarity, endosomal trafficking to the midbody and the assembly of the CEP55–ALIX–ESCRT complex (where ALIX is apoptosis-linked gene 2-interacting protein X and ESCRT is endosomal sorting complex required for transport) are drawn on opposite sides of the midbody. In reality, such mechanisms probably exist on both ‘sides’ of the midbody ring. a | During late telophase, RAB11- and RAB35-positive endosomes accumulate in the midbody by interacting with the vesicle-tethering exocyst complex, which is localized to the midbody ring by centriolin (not shown) and perhaps by interacting with BRUCE (see the main text). BRUCE is delivered to the midbody on membrane vesicles, on which it can also function as a diffusion barrier, preventing vesicle movement through the furrow. Collectively, these interactions anchor vesicles in the midbody. b | BRUCE is a ubiquitin ligase through which endosomal cargo might become ubiquitylated. CEP55 recruits ALIX and TSG101, which are components of ESCRTI, into the midbody. ESCRTII and ESCRTIII might also become localized here, perhaps through interactions with ALIX or TSG101, or with ubiquitylated endosomal cargo. These events might occur at the same time as vesicle docking (part a). c | ESCRT proteins form a lattice on endosomal membranes and induce membrane deformation. ESCRT complexes might also facilitate sorting away from midbody-localized endosomes, perhaps removing a ‘fusion brake’. The microtubule-severing protein spastin binds to the ESCRTIII subunit CHMP1B (charged multivesicular body protein 1B). These events (lattice formation and recruitment of spastin) might take place on endosomal surfaces, providing a degree of spatial resolution. In response to a signal, perhaps from an adjacent signalling complex, endosomes (and secretory vesicles) undergo compound fusion. CEP55 in the midbody ring might also function to recruit signalling proteins, such as Aurora B and Polo-like kinase 1 (PLK1), into this region. If spatially and temporally coupled to spastin cleavage of microtubules, this might constitute the basic machinery of abscission.
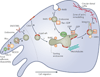
Figure 2. Endosome platforms in cell migration
The schematic shows a cell that is directing traffic towards circular dorsal ruffles (right) and at the same time disassembles focal adhesions at the rear of the cell (left). RAB5- and dynamin-dependent internalization of cell surface cargo (including Rac) into endosomes is shown (steps 1,2). The endosomes, which are characterized by RAB5, traffic Rac into an endosomal subcompartment that contains the Rac guanine nucleotide-exchange factor TiAM1, resulting in GTP loading, and activation, of Rac (step 3). Vesicles bud off of this compartment and contain activated RAC1 for forward-directed trafficking to the zone of ruffling (step 4). These vesicles might also contain integrin cargo and possibly lipid raft domains. The GTPase ARF6 has been implicated in the trafficking of both rafts and integrins, and hence it is possible that the intracellular endosomal compartment is a focal point for the assembly of an actin-remodelling ‘machine’ that traffics in an ARF6-dependent manner, possibly along microtubules towards the ruffles (step 5). Rafts are known to recycle through caveolae, which contain the protein caveolin 1 (CAV1; step 6). This recycling involves ARF6, suggesting a possible link between these two processes. Focal adhesion disassembly at the rear of the cell might also involve endosome platforms. The pro-migratory receptor ENDO180 (also known as MRC2) traffics between the cell surface and endosomes (step 7). On an appropriate cue, ENDO180 traffic is redirected into endosomes at the rear of the cell near focal adhesions, and serves as a platform for the assembly of Rho–ROCK1–MLC2-derived contractile signals (where MLC2 is myosin light chain 2 and ROCK1 is Rho-associated kinase 1) directly at sites of focal adhesions (steps 8,9).
Similar articles
- Subcellular localisation of retromer in post-endocytic pathways of polarised Madin-Darby canine kidney cells.
Mellado M, Cuartero Y, Brugada R, Verges M. Mellado M, et al. Biol Cell. 2014 Nov;106(11):377-93. doi: 10.1111/boc.201400011. Epub 2014 Sep 2. Biol Cell. 2014. PMID: 25081925 - Evidence for a sorting endosome in Arabidopsis root cells.
Jaillais Y, Fobis-Loisy I, Miège C, Gaude T. Jaillais Y, et al. Plant J. 2008 Jan;53(2):237-47. doi: 10.1111/j.1365-313X.2007.03338.x. Epub 2007 Nov 12. Plant J. 2008. PMID: 17999644 - Rab35 GTPase: A Central Regulator of Phosphoinositides and F-actin in Endocytic Recycling and Beyond.
Klinkert K, Echard A. Klinkert K, et al. Traffic. 2016 Oct;17(10):1063-77. doi: 10.1111/tra.12422. Epub 2016 Jul 14. Traffic. 2016. PMID: 27329675 Review. - The recycling endosome and its role in neurological disorders.
Li X, DiFiglia M. Li X, et al. Prog Neurobiol. 2012 May;97(2):127-41. doi: 10.1016/j.pneurobio.2011.10.002. Epub 2011 Oct 20. Prog Neurobiol. 2012. PMID: 22037413 Review. - Coats of endosomal protein sorting: retromer and ESCRT.
Schellmann S, Pimpl P. Schellmann S, et al. Curr Opin Plant Biol. 2009 Dec;12(6):670-6. doi: 10.1016/j.pbi.2009.09.005. Epub 2009 Oct 15. Curr Opin Plant Biol. 2009. PMID: 19836992 Review.
Cited by
- Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction.
Miranzadeh Mahabadi H, Taghibiglou C. Miranzadeh Mahabadi H, et al. Int J Mol Sci. 2020 Sep 25;21(19):7058. doi: 10.3390/ijms21197058. Int J Mol Sci. 2020. PMID: 32992764 Free PMC article. Review. - Biochemical and biological characterization of exosomes containing prominin-1/CD133.
Rappa G, Mercapide J, Anzanello F, Pope RM, Lorico A. Rappa G, et al. Mol Cancer. 2013 Jun 14;12:62. doi: 10.1186/1476-4598-12-62. Mol Cancer. 2013. PMID: 23767874 Free PMC article. - Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while Pannexin1 channels traffic to the plasma membrane.
Boassa D, Nguyen P, Hu J, Ellisman MH, Sosinsky GE. Boassa D, et al. Front Cell Neurosci. 2015 Feb 2;8:468. doi: 10.3389/fncel.2014.00468. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25698922 Free PMC article. - Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes.
Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, Heck AJ, Sheng M, Klumperman J, Rehmann H, Jaarsma D, Kapitein LC, van der Sluijs P. Hoogenraad CC, et al. PLoS Biol. 2010 Jan 19;8(1):e1000283. doi: 10.1371/journal.pbio.1000283. PLoS Biol. 2010. PMID: 20098723 Free PMC article. - Motif mimetic of epsin perturbs tumor growth and metastasis.
Dong Y, Wu H, Rahman HN, Liu Y, Pasula S, Tessneer KL, Cai X, Liu X, Chang B, McManus J, Hahn S, Dong J, Brophy ML, Yu L, Song K, Silasi-Mansat R, Saunders D, Njoku C, Song H, Mehta-D'Souza P, Towner R, Lupu F, McEver RP, Xia L, Boerboom D, Srinivasan RS, Chen H. Dong Y, et al. J Clin Invest. 2015 Dec;125(12):4349-64. doi: 10.1172/JCI80349. Epub 2015 Nov 16. J Clin Invest. 2015. PMID: 26571402 Free PMC article.
References
- Lemmon SK, Traub LM. Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol. 2000;12:457–466. - PubMed
- Christoforidis S, et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biol. 1999;1:249–252. - PubMed
- Gaullier J-M, et al. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. - PubMed
Publication types
MeSH terms
Grants and funding
- Z01 HD001609-14/ImNIH/Intramural NIH HHS/United States
- 13082/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/D000017/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- REI18423/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- Z01 HD001609/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources