Regulation of embryonic cell adhesion by the prion protein - PubMed (original) (raw)
Regulation of embryonic cell adhesion by the prion protein
Edward Málaga-Trillo et al. PLoS Biol. 2009.
Abstract
Prion proteins (PrPs) are key players in fatal neurodegenerative disorders, yet their physiological functions remain unclear, as PrP knockout mice develop rather normally. We report a strong PrP loss-of-function phenotype in zebrafish embryos, characterized by the loss of embryonic cell adhesion and arrested gastrulation. Zebrafish and mouse PrP mRNAs can partially rescue this knockdown phenotype, indicating conserved PrP functions. Using zebrafish, mouse, and Drosophila cells, we show that PrP: (1) mediates Ca(+2)-independent homophilic cell adhesion and signaling; and (2) modulates Ca(+2)-dependent cell adhesion by regulating the delivery of E-cadherin to the plasma membrane. In vivo time-lapse analyses reveal that the arrested gastrulation in PrP knockdown embryos is due to deficient morphogenetic cell movements, which rely on E-cadherin-based adhesion. Cell-transplantation experiments indicate that the regulation of embryonic cell adhesion by PrP is cell-autonomous. Moreover, we find that the local accumulation of PrP at cell contact sites is concomitant with the activation of Src-related kinases, the recruitment of reggie/flotillin microdomains, and the reorganization of the actin cytoskeleton, consistent with a role of PrP in the modulation of cell adhesion via signaling. Altogether, our data uncover evolutionarily conserved roles of PrP in cell communication, which ultimately impinge on the stability of adherens cell junctions during embryonic development.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
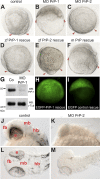
Figure 1. Differential Expression of PrP Genes in Zebrafish Embryos
The developmental expression patterns of PrP-1 and -2 were examined by whole mount in situ RNA hybridization using gene-specific probes. At mid-blastula stages (2.5 hpf, [A and C]), PrP-1 is ubiquitously transcribed at high levels and PrP-2 is not detectable. At pharyngula stages (30 hpf, [B and D]), low levels of PrP-1 transcripts appear restricted to the forebrain and eyes, while PrP-2 becomes strongly transcribed in defined neural structures. (A and C) show lateral views; (B and D) show dorsal views. d, diencephalon; llg, lateral line ganglion; nm, neuromeres; rb, Rohon-Beard sensory neurons; t, telencephalon; tg, trigeminal ganglion.
Figure 2. PrP Morpholino Knockdown and RNA Rescue in Zebrafish Embryos
(A–F) Embryos were microinjected at one- to four-cell stages and photographed at 9 hpf. (A) Control embryos reach approximately 90% epiboly, as evidenced by the normal progression of the blastodermal margin (red arrowheads). (B) Epiboly in PrP-1 morphant embryos is severely impaired, and they remain arrested. (C) No appreciable morphological defects are seen in PrP-2 morphant embryos. In rescue experiments, the morpholino (MO) PrP-1 phenotype is reverted to different extents by microinjection of PrP-1 (D), PrP-2 (E), or mouse PrP (F) mRNAs. m, mouse; zf, zebrafish. (G) Western blot (WB) analysis of 6-hpf embryo cell extracts shows that PrP-1 expression (∼53-kDa band) is effectively suppressed by morpholino injection. Co, control. (H and I) Embryos expressing the EGFP-PrP-1 fusion mRNA can overcome the MO PrP-1 arrest, normally reaching 75% epiboly at 8 hpf (H). In contrast, embryos expressing the corresponding control EGFP fusion mRNA remain arrested (I). (J–M) At the prim-5 (24 hpf) stage, PrP-2 morphant embryos (K and M) show severe malformations in the head region, compared to control embryos (J and L). (J and K), lateral views; (L and M), dorsal views. e, eye; fb, forebrain; hb, hindbrain; mb, hindbrain.
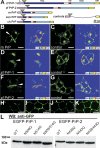
Figure 3. Expression of EGFP-Tagged PrPs in N2a Cells and in Zebrafish Embryos
(A) EGFP fluorescent constructs used in this study: the major domains of zebrafish (zf PrP-1 and -2), mouse (m PrP), Xenopus (xen PrP), and chick (ch PrP) prion proteins are indicated: leader peptide (L) in violet, repetitive region (Rep) in blue, hydrophobic domain (HD) in red, globular domain (Glob) in light blue, and GPI-anchored peptide (GPI) in yellow. Fluorescence tags are represented as green triangles; control constructs (controls) lack PrP cores (Rep, HD, and Glob); domains are shown as previously defined [5]. (B, D, and F) N2a cells transfected with mouse PrP (B), zebrafish PrP-1 (D) and PrP-2 (F) constructs show local accumulation of the fusion proteins at cell–cell contacts (white arrowheads and fluorescence profiles, right). (C, E, and G) The corresponding control EGFP fusion constructs are evenly distributed along the plasma membrane. (H–K) For visualization of PrP expression in zebrafish deep cells, embryos were microinjected with zebrafish PrP-1 (H), PrP-2 (I), mouse PrP (J), and PrP-1 (control [K]) EGFP fusion RNAs and analyzed at the sphere stage (4 hpf). (L) Predicted N-glycosylation sites of zf PrP-1 and -2 were confirmed by Western blot (WB) analysis (anti-GFP monoclonal antibody) of extracts from N2a cells transfected with the constructs indicated above each lane. WT, wild type. (B–G) show EGFP fluorescence (left), and total fluorescence profiles (right); (H–J) show EGFP fluorescence and Nomarski overlays. Scale bars in (B–G) indicate 10 μm; scale bars in (H–J) indicate 5 μm.
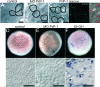
Figure 4. Effect of PrP-1 Knockdown in Embryonic Cell Adhesion
Differences in tissue compactness between deep cells of control, morphant, and rescued embryos were evaluated at the shield stage (6 hpf ). (A) Control embryos exhibit normal tissue compactness and polygonal cell shapes. (B) Reduced cell adhesion and rounded cells are evident in PrP-1 morphant (MO) embryos. (C) Local accumulation of EGFP-PrP-1 at cell contacts (see red arrowheads in detailed overlay view of framed region, right) reverts these effects in rescued embryos. Cell outlines were digitally redrawn to help visualization of cell shape. (D–F) The loss of embryonic cell adhesion is not related to cell-death, as whole-mount TUNEL stainings of control (D), morphant (E), and ethanol-treated embryos (F) show apoptotic cells (blue staining) only in (F). Scale bars in (A–C) indicate 10 μm; scale bars in (D–F) indicate 50 μm.
Figure 5. Aggregation and Cell Transplantation Assays Using PrP-1 Morphant Blastomeres
Shield stage (6 hpf) embryos were dissociated to single cells, the cells were allowed to reaggregate in suspension for 45 min and were then plated out for analysis. (A) Control embryos normally form small and large cell clusters. (B) Aggregation is considerably reduced in PrP-1 morphant cells. (C) When cocultured, PrP-1 morphant cells are generally excluded from control cell aggregates (white arrowhead). (D) Quantitative differences in aggregation potential were observed between PrP-1 morphant, control, and PrP-1 overexpressing cells in the presence and absence of Ca+2; the bar graph displays the ratios of loose, small (<10 cells), and large (>10 cells) aggregates in control (Co) and PrP-1 morphant (MO) cells, 45 min after dissociation. (E–G) Cell-autonomy of the PrP-1 defect was tested by embryonic cell transplantation: Labeled control deep cells (E) integrate into the control host tissue and resume polygonal shape. In contrast, PrP-1 morphant deep cells (F and G) fail to establish cell contacts with the control host cells. Adhesive properties were evaluated at 6 hpf, 2 h after transplantation. Scale bars in (A–C, E, and F) indicate 20 μm; scale bar in (G) indicates 100 μm.
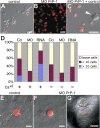
Figure 6. Regulation of Cadherin-Mediated Cell Adhesion by PrP-1
Differences in the subcellular distribution of adherens junction components between control and PrP-1 morphant (MO) embryos were evaluated in the deep cell layer at the shield stage (6 hpf). (A–F) The normal membrane localization of E-cadherin (E-cad [A]), β-catenin (β-cat [B]), and cortical F-actin (C) in control embryos appears disrupted upon PrP-1 knockdown (D–F). (G) Western blot (WB) analysis of embryo cell extracts (6 hpf) reveals an almost complete reduction in the relative levels of mature E-cadherin (120 kDa, red arrowhead), and a slight increase in the levels of its immature form (140 kDa, black arrowhead) upon PrP-1 knockdown. γ-Tub, γ-tubulin. (H–M) Changes in the number of Rab11-positive vesicles containing E-cadherin between control (Co [H–J]) and PrP-1 morphant embryos (K–M) were analyzed by immunostaining. Compared to control embryos, PrP-1 morphant embryos exhibit a higher density of E-cadherin/Rab11 double-positive vesicles in deep cells (white circles in [J and M]). (N) The quantitative difference in the number of E-cadherin/Rab11 colocalizations per cell (_y_-axis) between control (Co) and PrP-1 morphant embryos (MO) was statistically significant for EVL (p = 0.0002) and DC (deep cells, p = 0.0004); triple asterisks (***) indicate statistical significance at p < 0.001. Error bars indicate SEM. (O–V) Accumulation of E-cadherin (O), β-catenin (P), Fyn tyrosine kinase (Q), and phosphotyrosine staining (R) at cell contacts between primary blastomeres derived from control embryos (white arrowheads) is lost in PrP-1 morphant blastomeres (S, T, U, and V). Scale bars in (A–F) indicate 10 μm; scale bars in (H–M and O–V) indicate 5 μm.
Figure 7. Radial Intercalation in Control and PrP-1 Morphant Embryos
Cell movements in lateral regions of the epiblast (8 hpf) were imaged over 20 min using Nomarski optics. (Time in minutes and seconds is shown in the lower-right corner of each panel.) Selected frames from time-lapse video recordings were pseudocolored: intercalating and surrounding cells are shown in blue and green, respectively (after Kane et al. [22]), and cells forming new contacts are shown in red. (A) In control embryos, cells from the interior layer (blue) intercalate among cells in the exterior layer (green) and establish additional cell contacts with other cells (red). (B) In PrP-1 morphant embryos, cells from the interior layer often intercalate and then deintercalate, thereby failing to maintain attachment to cells from the exterior layer (green cells 2 and 4, and red cells).
Figure 8. PrP-1 Regulation of Adherens, but Not Tight Junctions, in EVL Cells
Differences in the subcellular distribution of various cell junction components between control and PrP-1 morphant embryos (MO) were evaluated in the polarized epithelial cells of the EVL at the shield stage (6 hpf). In control embryos, a marked membrane localization pattern can be seen for adherens junction (E-cadherin [E-cad], β-catenin [β-cat], and F-actin) (A–C) and tight junction markers (Occludin and ZO-1) (D and E). PrP-1 knockdown induces partial mislocalization of adherens junction components (F–H) but does not affect the distribution of classical tight junction markers (I and J). Scale bars indicate 20 μm.
Figure 9. Cell Signaling and Adhesion in Drosophila S2 Cells upon PrP Expression
(A–E) Expression of mouse PrP (m PrP [A]), zebrafish PrP-1 (zf PrP-1 [B]) and PrP-2 (zf PrP-2 [C]), Xenopus PrP (xen PrP [D]), and chick PrP (ch PrP [E]) EGFP fusion constructs in Drosophila nonadhesive S2 cells results in the induction of cell–cell contact formation and local PrP accumulation at cell contacts (white arrowheads). (F) Control EGFP fusion constructs do not induce this phenomenon (shown for m PrP at a fortuitous PrP-independent cell contact). (G) Cell contact formation and PrP accumulation (white arrowheads) in mixed S2 cell populations separately transfected with mouse EGFP- and DsRed-monomer-PrP constructs (G) exclude cell division artifacts. (H) The same result is obtained when mouse DsRed-monomer-PrP and zebrafish EGFP-PrP-2 constructs are used, suggesting PrP interaction across species. (I) Blocking of mouse PrP–mediated aggregation of S2 cells (red arrows, lower panel) by a polyclonal antibody against mouse PrP (M-20) shows that the formation of cell clusters (white arrowheads, upper left panel) is specifically induced by PrP. The effect was quantified as the number of cell contacts between S2 cells expressing mouse PrP in the absence (upper left) or presence (upper right) of M-20 or a control antibody at the concentrations indicated in the graph (double asterisks [**] indicate statistical significance at p < 0.01, one-way ANOVA test; error bars indicate SEM). (J–O) Strong anti–phospho-Src kinase immunostaining ([J] α-pSrc), as well as accumulation of rat reggie-1-DsRed-monomer ([L] reggie-1) and Alexa-568 Phalloidin ([N] F-actin) colocalize at mo PrP–mediated cell contacts (white arrowheads), but not at fortuitous PrP-independent cell contacts (K, M, and O). Scale bars in (A–H and J–O) indicate 5 μm; scale bar in (I) indicates 20 μm.
Similar articles
- Activation of zebrafish Src family kinases by the prion protein is an amyloid-β-sensitive signal that prevents the endocytosis and degradation of E-cadherin/β-catenin complexes in vivo.
Sempou E, Biasini E, Pinzón-Olejua A, Harris DA, Málaga-Trillo E. Sempou E, et al. Mol Neurodegener. 2016 Feb 9;11:18. doi: 10.1186/s13024-016-0076-5. Mol Neurodegener. 2016. PMID: 26860872 Free PMC article. - Conserved roles of the prion protein domains on subcellular localization and cell-cell adhesion.
Solis GP, Radon Y, Sempou E, Jechow K, Stuermer CA, Málaga-Trillo E. Solis GP, et al. PLoS One. 2013 Jul 31;8(7):e70327. doi: 10.1371/journal.pone.0070327. Print 2013. PLoS One. 2013. PMID: 23936187 Free PMC article. - Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation.
Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, Fujita Y, Wilson SW, Tada M. Carreira-Barbosa F, et al. Development. 2009 Feb;136(3):383-92. doi: 10.1242/dev.026542. Epub 2008 Dec 17. Development. 2009. PMID: 19091770 Free PMC article. - PrPs: Proteins with a purpose: Lessons from the zebrafish.
Málaga-Trillo E, Sempou E. Málaga-Trillo E, et al. Prion. 2009 Jul-Sep;3(3):129-33. doi: 10.4161/pri.3.3.9651. Epub 2009 Jul 29. Prion. 2009. PMID: 19786844 Free PMC article. Review. - Adherens and tight junctions: structure, function and connections to the actin cytoskeleton.
Hartsock A, Nelson WJ. Hartsock A, et al. Biochim Biophys Acta. 2008 Mar;1778(3):660-9. doi: 10.1016/j.bbamem.2007.07.012. Epub 2007 Jul 27. Biochim Biophys Acta. 2008. PMID: 17854762 Free PMC article. Review.
Cited by
- Physiology of Cellular Prion Proteins in Reproduction.
Svedružić ŽM, Ryou C, Choi D, Lee SH, Cheon YP. Svedružić ŽM, et al. Dev Reprod. 2024 Jun;28(2):29-36. doi: 10.12717/DR.2024.28.2.29. Epub 2024 Jun 30. Dev Reprod. 2024. PMID: 39055100 Free PMC article. - Comparing Prion Proteins Across Species: Is Zebrafish a Useful Model?
Burato A, Legname G. Burato A, et al. Mol Neurobiol. 2024 Jun 25. doi: 10.1007/s12035-024-04324-z. Online ahead of print. Mol Neurobiol. 2024. PMID: 38918277 Review. - The role of cellular prion protein in immune system.
Cha S, Kim MY. Cha S, et al. BMB Rep. 2023 Dec;56(12):645-650. doi: 10.5483/BMBRep.2023-0151. BMB Rep. 2023. PMID: 37817440 Free PMC article. Review. - Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts.
Matamoros-Angles A, Mohammadi B, Song F, Shafiq M, Brenna S, Puig B, Glatzel M, Altmeppen HC. Matamoros-Angles A, et al. Neural Regen Res. 2023 Sep;18(9):1869-1875. doi: 10.4103/1673-5374.366496. Neural Regen Res. 2023. PMID: 36926701 Free PMC article. Review. - Melatonin: Regulation of Prion Protein Phase Separation in Cancer Multidrug Resistance.
Loh D, Reiter RJ. Loh D, et al. Molecules. 2022 Jan 21;27(3):705. doi: 10.3390/molecules27030705. Molecules. 2022. PMID: 35163973 Free PMC article. Review.
References
- Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. - PubMed
- Collinge J. Human prion diseases and bovine spongiform encephalopathy (BSE) Hum Mol Genet. 1997;6:1699–1705. - PubMed
- Harris DA. Trafficking, turnover and membrane topology of PrP. Br Med Bull. 2003;66:71–85. - PubMed
- Aguzzi A, Baumann F, Bremer J. The Prion's elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. - PubMed
- Rivera-Milla E, Oidtmann B, Panagiotidis CH, Baier M, Sklaviadis T, et al. Disparate evolution of prion protein domains and the distinct origin of Doppel- and prion-related loci revealed by fish-to-mammal comparisons. FASEB J. 2006;20:317–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous