CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells - PubMed (original) (raw)
Review
CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells
Michelle L Hermiston et al. Immunol Rev. 2009 Mar.
Erratum in
- Immunol Rev. 2009 May;229(1):387
Abstract
Reciprocal regulation of tyrosine phosphorylation by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) is central to normal immune cell function. Disruption of the equilibrium between PTK and PTP activity can result in immunodeficiency, autoimmunity, or malignancy. Src family kinases (SFKs) play a central role in both immune cell function and disease due to their proximal position in numerous signal transduction cascades including those emanating from integrin, T and B-cell antigen receptors, Fc, growth factor, and cytokine receptors. Given that tight regulation of SFKs activity is critical for appropriate responses to stimulation of these various signaling pathways, it is perhaps not surprising that multiple PTPs are involved in their regulation. Here, we focus on the role of three phosphatases, CD45, CD148, and LYP/PEP, which are critical regulators of SFKs in hematopoietic cells. We review our current understanding of their structures, expression, functions in different hematopoietic cell subsets, regulation, and putative roles in disease. Finally, we discuss remaining questions that must be addressed if we are to have a clearer understanding of the coordinated regulation of tyrosine phosphorylation and signaling networks in hematopoietic cells and how they could potentially be manipulated therapeutically in disease.
Figures
Figure 1. SFKs are in a dynamic equilibrium between closed and active conformations
Interaction of the SH2 domain and the C-terminal negative regulatory phosphotyrosine results in a closed conformation with decreased access to the kinase's catalytic site. Dephosphorylation of this site results in adoption of a more open, or ‘primed’, conformation. This conformation can potentially also be facilitated when the relatively weak interaction between the SH2 domain and the C-terminal phosphotyrosine is overcome by competing interactions of other proteins (denoted in the schematic as X or Y) with the SH2 or SH3 domains of the SFK. Phosphorylation of the catalytic site tyrosine is required for full kinase activity. (Adapted from (2,7)).
Figure 2. CD45, CD148, and Lyp/Pep structures
Key functional domains are depicted. Both CD45 and CD148 are RPTPs while Lyp/Pep lacks a transmembrane domain and is cytoplasmic. CD45 exists as multiple isoforms due to alternative splicing of exons 4, 5, and 6 that encode three regions, designated A, B, and C, in the extracellular domain, which contain multiple sites for O-linked glycosylation and sialyation. The largest (RABC) and smallest (RO) isoforms are shown. While CD45 contains two PTP domains (of which only D1 is active), CD148 and Lyp/Pep contain only a single PTP domain.
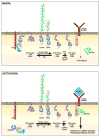
Figure 3. Reciprocal regulation of SFK activation by CD45, CD148, and Lyp/Pep
SFKs are in a dynamic equilibrium between their inactive and active conformations in both the basal state and during immune cell activation (while B cell activation is depicted, similar mechanisms likely exist in other hematopoietic cell lineages). In the basal state, phosphorylation of the adapter PAG facilitates recruitment of Csk and Lyp/Pep. Csk phosphorylates the negative regulatory tyrosine while Lyp/Pep dephosphorylates the autocatalytic tyrosine. This results in the SFK adopting a closed conformation. By opposing Csk and dephosphorylating the negative regulatory tyrosine, CD45 and CD148 generate a pool of SFKs in an open or ‘primed’ conformation that can be rapidly activated should antigen be encountered. We hypothesize that low levels of SFK transphosphorylation of the autocatalytic site occur in the basal state, which is necessary for tonic signaling and cell survival. This is balanced by the action of PAG/Csk/Pep (and potentially CD45 targeting either directly or indirectly the autocatalytic tyrosine). During antigen encounter and receptor activation, PAG becomes dephosphorylated via mechanisms that are incompletely elucidated (but may involve CD45), Csk and Lyp/Pep dissociate from the cell surface leaving CD45 and CD148 actions on the negative regulatory tyrosine unopposed. Phosphorylation of the autocatalytic tyrosine is now favored. The fully active SFK can phosphorylate the ITAMs of the immunoreceptor, facilitating recruitment of Syk/Zap70 and amplification of downstream signaling cascades.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - "I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.
Huang Y, Trollor JN, Foley KR, Arnold SRC. Huang Y, et al. Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article. - Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.
Tweed E, Cimova K, Craig P, Allik M, Brown D, Campbell M, Henderson D, Mayor C, Meier P, Watson N. Tweed E, et al. Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242 - Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits.
Galea CA, Wang Y, Sivakolundu SG, Kriwacki RW. Galea CA, et al. Biochemistry. 2008 Jul 22;47(29):7598-609. doi: 10.1021/bi8006803. Biochemistry. 2008. PMID: 18627125 Free PMC article. Review.
Cited by
- The chemical biology of protein hydropersulfides: Studies of a possible protective function of biological hydropersulfide generation.
Millikin R, Bianco CL, White C, Saund SS, Henriquez S, Sosa V, Akaike T, Kumagai Y, Soeda S, Toscano JP, Lin J, Fukuto JM. Millikin R, et al. Free Radic Biol Med. 2016 Aug;97:136-147. doi: 10.1016/j.freeradbiomed.2016.05.013. Epub 2016 May 27. Free Radic Biol Med. 2016. PMID: 27242269 Free PMC article. - Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival.
Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Zikherman J, et al. Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):E3-12. doi: 10.1073/pnas.1117374108. Epub 2011 Nov 30. Proc Natl Acad Sci U S A. 2012. PMID: 22135465 Free PMC article. - Modulation of tight junction structure and function by kinases and phosphatases targeting occludin.
Dörfel MJ, Huber O. Dörfel MJ, et al. J Biomed Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. Epub 2012 Jan 23. J Biomed Biotechnol. 2012. PMID: 22315516 Free PMC article. Review. - The Mouse Cytomegalovirus Gene m42 Targets Surface Expression of the Protein Tyrosine Phosphatase CD45 in Infected Macrophages.
Thiel N, Keyser KA, Lemmermann NA, Oduro JD, Wagner K, Elsner C, Halenius A, Lenac Roviš T, Brinkmann MM, Jonjić S, Cicin-Sain L, Messerle M. Thiel N, et al. PLoS Pathog. 2016 Dec 7;12(12):e1006057. doi: 10.1371/journal.ppat.1006057. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27926943 Free PMC article. - B-cell tolerance and autoimmunity.
Tsubata T. Tsubata T. F1000Res. 2017 Mar 29;6:391. doi: 10.12688/f1000research.10583.1. eCollection 2017. F1000Res. 2017. PMID: 28408984 Free PMC article. Review.
References
- Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008 Sep;8(9):699–712. - PubMed
- Vang T, Miletic AV, Arimura Y, Tautz L, Rickert RC, Mustelin T. Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol. 2008;26:29–55. - PubMed
- Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004 Jul;41(67):631–43. - PubMed
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004 Oct 18;23(48):7990–8000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI035297-17/AI/NIAID NIH HHS/United States
- K08 CA098418-03/CA/NCI NIH HHS/United States
- P01 AI035297-170007/AI/NIAID NIH HHS/United States
- P01 AI035297/AI/NIAID NIH HHS/United States
- K08 CA098418/CA/NCI NIH HHS/United States
- P01 AI355297/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous