MicroRNA-125b is a novel negative regulator of p53 - PubMed (original) (raw)
MicroRNA-125b is a novel negative regulator of p53
Minh T N Le et al. Genes Dev. 2009.
Abstract
The p53 transcription factor is a key tumor suppressor and a central regulator of the stress response. To ensure a robust and precise response to cellular signals, p53 gene expression must be tightly regulated from the transcriptional to the post-translational levels. Computational predictions suggest that several microRNAs are involved in the post-transcriptional regulation of p53. Here we demonstrate that miR-125b, a brain-enriched microRNA, is a bona fide negative regulator of p53 in both zebrafish and humans. miR-125b-mediated down-regulation of p53 is strictly dependent on the binding of miR-125b to a microRNA response element in the 3' untranslated region of p53 mRNA. Overexpression of miR-125b represses the endogenous level of p53 protein and suppresses apoptosis in human neuroblastoma cells and human lung fibroblast cells. In contrast, knockdown of miR-125b elevates the level of p53 protein and induces apoptosis in human lung fibroblasts and in the zebrafish brain. This phenotype can be rescued significantly by either an ablation of endogenous p53 function or ectopic expression of miR-125b in zebrafish. Interestingly, miR-125b is down-regulated when zebrafish embryos are treated with gamma-irradiation or camptothecin, corresponding to the rapid increase in p53 protein in response to DNA damage. Ectopic expression of miR-125b suppresses the increase of p53 and stress-induced apoptosis. Together, our study demonstrates that miR-125b is an important negative regulator of p53 and p53-induced apoptosis during development and during the stress response.
Figures
Figure 1.
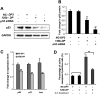
miR-125b binds to the 3′ UTR of zebrafish and human p53 mRNAs. (A) Outline of luciferase reporter assay for validating the interaction of miR-125b with the 3′ UTR of p53: The MREs of miR-125b in the 3′ UTR of human and zebrafish p53 mRNA were predicted by TargetScan and miRBase Target. Shaded texts indicate the “seed” regions. Each predicted MRE or the whole p53 3′ UTR was inserted into a psiCheck2 vector, immediately downstream from the Renilla luciferase gene. In mutant reporter constructs, the MRE was deleted or a three-mismatch mutation was introduced into the seed region. Each luciferase construct was cotransfected with negative control duplex 1 (NC-DP1) or miR-125b duplex (125b-DP) into HEK-293T cells, and luciferase readings were obtained 48 h after transfection. (B) Repression of luciferase activity due to the interaction between miR-125b and the predicted MREs in the _luciferase_-MRE or in the _luciferase–p53_–3′ UTR constructs. Repression was abolished when the MRE was deleted or mutated. Every Renilla luciferase reading was normalized to that of the control firefly luciferase. The luciferase activities of 125b-DP-transfected cells were presented as percentages relative to the level of luciferase in the NC-DP1-transfected cells (this control luciferase level is considered as 100% and is represented by the solid red line). The values represent average ± SEM (n ≥ 6). The dashed line represents the threshold of luciferase activity (75%), suppression of luciferase level below which indicates positive binding. Two-tail _t_-test results are indicated by (*) P < 0.05 and (**) P < 0.01, relative to the NC-DP1-transfected controls. (C) Overexpression of miR-125b down-regulates the wild-type but not the MRE-deleted human p53 (hp53) in H1299 cells: pCDNA3.1+ vector containing the full-length human p53 cDNA sequence with or without the miR-125b MRE was transfected alone or cotransfected with negative control duplex 3 (NC-DP3) or 125b-DP into H1299 cells. The level of human p53 was analyzed by Western blots 2 d after transfection. (D) Overexpression of miR-125b down-regulates the wild-type but not the MRE-deleted zebrafish p53 (fp53) in H1299 cells: pCDNA3.1+ vector containing the full-length zebrafish p53 cDNA sequence with or without the MRE of miR-125b was transfected alone or cotransfected with NC-DP3 or 125b-DP into H1299 cells. The level of zebrafish p53 was analyzed by Western blots 2 d after transfection. (E) p53 protein level was quantified from the Western blot bands in C and D, normalized to GAPDH level, and presented as fold change ± SEM (n ≥ 3) relative to the p53 level of p53-only-transfected cells (solid red line). The dashed line represents the threshold of suppression (0.75-fold) corresponding to threshold set in the luciferase reporter assay. Two-tail _t_-test results are indicated by (*) P < 0.05 and (**) P < 0.01, relative to the _p53_-only-transfected controls.
Figure 2.
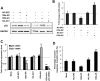
miR-125b represses the endogenous p53 expression and suppresses p53-induced apoptosis in human neuroblastoma SH-SY5Y cells. (A) The endogenous p53 protein level in SH-SY5Y cells 2 d after a transfection with mock (water), negative control duplex 3 (NC-DP3), miR-125b duplex (125b-DP), or p53 siRNA. (B) The p53 protein level was quantified from the Western blot bands in A, normalized to the GAPDH level, and presented as fold change ± SEM (n ≥ 3) relative to the p53 level of mock-transfected cells. (C) The mRNA expression levels of p53, p21, and bax in SH-SY5Y cells 2 d after transfection with NC-DP1 or 125b-DP. The expression was quantified by real-time PCR, normalized to the expression of β-actin, and presented as fold change ± SEM (n ≥ 4) relative to that in the cells transfected with NC-DP1. (D) The percentage of SH-SY5Y cells positive for active caspase-3 was quantified by the Cellomics high-content screening system 2 d after a transfection with NC-DP1 or with 125b-DP. The 10 μM H-7 treatment was applied 24 h before fixing. The values represent average ± SEM (n ≥ 3). For each replicate, 20 images (including at least 10,000 cells) were analyzed. In all panels, two-tail _t_-test results are indicated by (*) P < 0.05 and (**) P < 0.01, relative to the mock-transfected or NC-DP-transfected controls.
Figure 3.
miR-125b represses the endogenous p53 expression and suppresses apoptosis in human lung fibroblast cells. (A) The endogenous p53 level in human lung fibroblast cells 2 d after transfection with mock (water), negative control duplex 2 (NC-DP2), or miR-125b duplex (125b-DP); and 1 d after transfection with mock, negative control antisense 1 (NC-AS1), or miR-125b antisense (125b-AS). (B) The p53 protein level was quantified from the Western blot bands in A, normalized to the GAPDH level and presented as fold change ± SEM (n ≥ 3) relative to the p53 level of mock-transfected cells (dotted line). (C) The levels of p53 mRNA and p21 mRNA in human lung fibroblast cells 2 d after transfection with mock, NC-DP2, 125b-DP, or NC-AS1,125b-AS, or a cotransfection of 125b-AS and p53 siRNA. The expression was quantified by real-time PCR, normalized to the expression of β-actin and presented as fold change ± SEM (n ≥ 4) relative to that in the mock-transfected cells (dotted line). (D) The percentage of human lung fibroblast cells positive for active caspase-3, 2 d after transfection with mock, NC-DP2, 125b-DP, NC-AS1, or 125b-AS was quantified by the Cellomics high-content screening system. The values represent average ± SEM (n ≥ 3). For each replicate, 20 images (including at least 10,000 cells) were analyzed. In all panels, two-tail _t_-test results are indicated by (*) P < 0.05 and (**) P < 0.01, relative to the mock-transfected controls.
Figure 4.
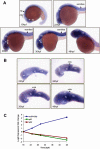
Spatio-temporal expression of miR-125b during zebrafish embryogenesis. (A) Whole-mount in situ hybridization of miR-125b in zebrafish embryos at 19 hpf, 22 hpf, 26 hpf, 30 hpf, and 48 hpf. Side view of the whole body excluding the tail is shown. (B) Side view of zebrafish brain, in situ hybridization with miR-125b at 22 hpf, 26 hpf, 30 hpf, and 48 hpf. In A and B, each image shows the expression pattern of miR-125b in a representative embryo. The same pattern was observed in all 20 embryos examined at each developmental stage. (ey) Eye; (hb) hindbrain; (hyp) hypothalamus; (mhb) midbrain–hindbrain boundary; (ot) optic tectum; (tel) telencephalon; (tg) tegmentum. (C) The expression pattern of miR-125b, p53, and p21 during zebrafish development: Transcript levels were quantified by real-time PCR, normalized to internal controls (18S or β-actin), and presented as log2 fold change ± SEM (n ≥ 4) relative to the expression at 18 hpf.
Figure 5.
Loss of miR-125b in zebrafish embryos. (A) Design of morpholinos targeting either the guide strand of mature miR-125b (m125b) or the loop regions of pre-mir-125b (lp125b). Three different lp125b morpholinos (lp125bMO1/2/3) were designed for the three isoforms of pre-mir-125b. (B) qRT–PCR elucidating the effects of miR-125b morpholinos on the endogenous level of zebrafish miR-125b at 24 hpf. One-cell-stage embryos were injected with m125bMO or lp125bMO1/2/3 (individually or together). A morpholino (misMO) with 5 nt different from m125bMO was used as control. Total RNA was obtained from the embryos at 24 hpf. All the expression values were normalized to 18S RNA levels and presented as average percentage ± SEM (n ≥ 4) relative to the expression values in uninjected controls. Two-tail _t_-test results are indicated by (**) P < 0.01, relative to the uninjected control. (C) Loss-of-function morphology at 24 hpf: Morphants typically exhibit severe cell death in the brain (brackets), absence of the midbrain–hindbrain boundary (*), smaller eyes (blue arrows), and deformed somites (green arrows). Each control/morphant embryo is shown with a lateral view of the whole body and a magnified view of the head. The total number of embryos (n) in each treatment and the percentage of embryos having the same phenotype as in the representative picture are indicated below each image.
Figure 6.
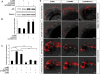
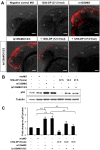
Loss of miR-125b elevates p53 and triggers p53-dependent apoptosis in zebrafish embryos. (A) Elevation of p53 protein caused by loss of miR-125b in zebrafish embryos: Embryos were injected with misMO, m125bMO, or lp125bMO1/2/3. Western blotting was performed at 24 hpf. (B) The p53 protein level was quantified from the Western blot bands in A, normalized to tubulin level, and presented as fold change ± SEM (n ≥ 3) relative to the p53 level in the misMO-injected embryos. Two-tail _t_-test results are indicated by (**) P < 0.01, relative to the misMO-injected control. (C) qRT–PCR of p21 transcripts at 24 hpf in embryos injected with different combinations of morpholinos. p53MO indicates a morpholino blocking translation of p53. The values were normalized to the expression level of β-actin and represented as average fold change ± SEM (n ≥ 4) relative to the expression level in misMO-injected embryos (dashed line). Two-tail _t_-test results are indicated as (**) P < 0.01. (D) TUNEL assay for detecting apoptotic cells (visualized as red spots) in the 24-hpf brains and acetylated tubulin staining (αAT) marking mature neurons and axonal tracts in the 48-hpf brains of wild-type and p53M214K mutant embryos microinjected with misMO, m125bMO, or lp125bMO1/2/3. Each image is a projection of multiple optical slides obtained from a representative embryo. Three embryos were observed for each condition for the TUNEL assay, and five were observed for each condition in the αAT staining. All of them had a similar phenotype as the representative images. (AC) Anterior commissure; (d) diencephalon; (fb) forebrain; (hb) hindbrain; (mb) midbrain; (MLF) medial longitudinal fasciculus; (ot) optic tectum; (SOT) supraoptic tract; (TPC) tract of posterior commissure; (TPOC) tract of postoptic commissure; (t) telencephalon. Bar, 50 μm.
Figure 7.
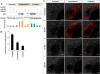
Synthetic miR-125b rescues apoptosis in miR-125b morphants. (A) TUNEL assay for detecting apoptotic cells in the 24-hpf brains: Embryos were injected with a standard negative control morpholino, miR-125b duplex (125b-DP), m125bMO, or lp125bMO1/2/3. Two different concentrations of 125b-DP (12.5 fmol and 37.5 fmol per injection) were used to rescue the embryos injected with lp125bMO1/2/3. Each image is a projection of multiple optical slides from a representative embryo. Three embryos were observed for each condition, and all of them had a similar phenotype as the representative images. (fb) Forebrain; (hb) hindbrain; (mb) midbrain. Bar, 50 μm. (B) Regulation of p53 protein in the morphants and the rescued embryos. Western blotting was performed at 24 hpf. (C) p53 protein level was quantified from the Western blot bands in B, normalized to tubulin level, and presented as fold change ± SEM (n ≥ 3) relative to the p53 level in the embryos injected with misMO. Two-tail _t_-test results are indicated by (**) P < 0.01.
Figure 8.
Overexpression of miR-125b rescues stress-induced apoptosis. (A) Regulation of p53 protein in zebrafish embryos injected with negative control duplex 1 (NC-DP1), p53 morpholino (p53 MO), or miR-125b duplex (125b-DP). At 24 hpf, uninjected and injected embryos were treated with 500 nM camptothecin for 8 h or subjected to 40 Gy of γ-irradiation. Protein lysate from the two treatments with two sets of untreated control were loaded on two separate gels. The bar chart presents quantification of p53 Western blot band intensity, normalized to tubulin levels, and presented as fold change relative to the uninjected untreated control of each blot. (B) Regulation of miR-125b in uninjected embryos or those treated with 500 nM camptothecin for 8 h or subjected to 40 Gy of γ-irradiation, normalized to 18S RNA level, and presented as average fold change relative to untreated control ± SEM (n ≥ 6). Two-tail _t_-test results are indicated as (**) P < 0.01, relative to the untreated control. (C) Staining of apoptotic cells in embryos uninjected or injected with NC-DP1, p53 MO, or 125b-DP, treated with 500 nM camptothecin for 8 h or with 40 Gy of γ-irradiation. Embryos were fixed at 32 hpf and subjected to TUNEL assay. Each image is a projection of multiple optical slides from a representative embryo. Three embryos were observed for each condition, and all of them had a similar phenotype as in the representative image. (fb) Forebrain; (hb) hindbrain; (mb) midbrain. Bar, 50 μm.
Similar articles
- Conserved regulation of p53 network dosage by microRNA-125b occurs through evolving miRNA-target gene pairs.
Le MT, Shyh-Chang N, Khaw SL, Chin L, Teh C, Tay J, O'Day E, Korzh V, Yang H, Lal A, Lieberman J, Lodish HF, Lim B. Le MT, et al. PLoS Genet. 2011 Sep;7(9):e1002242. doi: 10.1371/journal.pgen.1002242. Epub 2011 Sep 15. PLoS Genet. 2011. PMID: 21935352 Free PMC article. - Interplay between RNA-binding protein HuR and microRNA-125b regulates p53 mRNA translation in response to genotoxic stress.
Ahuja D, Goyal A, Ray PS. Ahuja D, et al. RNA Biol. 2016 Nov;13(11):1152-1165. doi: 10.1080/15476286.2016.1229734. Epub 2016 Sep 3. RNA Biol. 2016. PMID: 27592685 Free PMC article. - p53 and HuR combinatorially control the biphasic dynamics of microRNA-125b in response to genotoxic stress.
Goswami B, Ahuja D, Pastré D, Ray PS. Goswami B, et al. Commun Biol. 2023 Jan 27;6(1):110. doi: 10.1038/s42003-023-04507-9. Commun Biol. 2023. PMID: 36707647 Free PMC article. - Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways.
Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY, Zhang H, Chen YQ. Zeng CW, et al. Mol Pharmacol. 2012 Apr;81(4):578-86. doi: 10.1124/mol.111.076794. Epub 2012 Jan 17. Mol Pharmacol. 2012. PMID: 22252650 - Negative regulation of tumor suppressor p53 by microRNA miR-504.
Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS, Tang LH, Levine AJ, Feng Z. Hu W, et al. Mol Cell. 2010 Jun 11;38(5):689-99. doi: 10.1016/j.molcel.2010.05.027. Mol Cell. 2010. PMID: 20542001 Free PMC article.
Cited by
- Unveiling the principle of microRNA-mediated redundancy in cellular pathway regulation.
Fischer S, Handrick R, Aschrafi A, Otte K. Fischer S, et al. RNA Biol. 2015;12(3):238-47. doi: 10.1080/15476286.2015.1017238. RNA Biol. 2015. PMID: 25826657 Free PMC article. - MicroRNA regulation in extreme environments: differential expression of microRNAs in the intertidal snail Littorina littorea during extended periods of freezing and anoxia.
Biggar KK, Kornfeld SF, Maistrovski Y, Storey KB. Biggar KK, et al. Genomics Proteomics Bioinformatics. 2012 Oct;10(5):302-9. doi: 10.1016/j.gpb.2012.09.002. Epub 2012 Oct 8. Genomics Proteomics Bioinformatics. 2012. PMID: 23200140 Free PMC article. - Role of microRNA processing in adipose tissue in stress defense and longevity.
Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, Kahn CR. Mori MA, et al. Cell Metab. 2012 Sep 5;16(3):336-47. doi: 10.1016/j.cmet.2012.07.017. Cell Metab. 2012. PMID: 22958919 Free PMC article. - Signaling pathways involved in colorectal cancer: pathogenesis and targeted therapy.
Li Q, Geng S, Luo H, Wang W, Mo YQ, Luo Q, Wang L, Song GB, Sheng JP, Xu B. Li Q, et al. Signal Transduct Target Ther. 2024 Oct 7;9(1):266. doi: 10.1038/s41392-024-01953-7. Signal Transduct Target Ther. 2024. PMID: 39370455 Free PMC article. Review. - MicroRNAs Targeting Critical Molecular Pathways in Diabetic Cardiomyopathy Emerging Valuable for Therapy.
Mathur P, Saxena S, Saxena B, Rani V. Mathur P, et al. Cardiovasc Hematol Agents Med Chem. 2024;22(3):298-307. doi: 10.2174/0118715257265947231129074526. Cardiovasc Hematol Agents Med Chem. 2024. PMID: 38265401 Review.
References
- Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
- Bernstein E., Kim S.Y., Carmell M.A., Murchison E.P., Alcorn H., Li M.Z., Mills A.A., Elledge S.J., Anderson K.V., Hannon G.J. Dicer is essential for mouse development. Nat. Genet. 2003;35:215–217. - PubMed
- Bloomston M., Frankel W.L., Petrocca F., Volinia S., Alder H., Hagan J.P., Liu C.G., Bhatt D., Taccioli C., Croce C.M. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK47636/DK/NIDDK NIH HHS/United States
- R01 DK047636/DK/NIDDK NIH HHS/United States
- AI54973/AI/NIAID NIH HHS/United States
- R01 AI054973/AI/NIAID NIH HHS/United States
- R01 DK068348/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous