Adoptive cell therapy for the treatment of patients with metastatic melanoma - PubMed (original) (raw)
Review
Adoptive cell therapy for the treatment of patients with metastatic melanoma
Steven A Rosenberg et al. Curr Opin Immunol. 2009 Apr.
Abstract
Adoptive cell therapy (ACT) is the best available treatment for patients with metastatic melanoma. In a recent series of three consecutive clinical trials using increasing lymphodepletion before infusion of autologous tumor infiltrating lymphocytes (TIL), objective response rates between 49% and 72% were seen. Persistence of infused cells in the circulation at one month was highly correlated with anti-tumor response as was the mean telomere length of the cells infused and the number of CD8+ CD27+ cells infused. Responses occur at all sites and appear to be durable with many patients in ongoing response beyond three years. In the most recent trial of 25 patients receiving maximum lymphodepletion, seven of the 25 patients (28%) achieved a complete response. Of the 12 patients in the three trials who achieved a complete response all but one are ongoing between 18 and 75 months. We recently demonstrated that ACT using autologous lymphocytes genetically modified to express anti-tumor T cell receptors can mediate tumor regression and this approach is now being applied to patients with common epithelial cancers.
Figures
Figure 1
Schema of the lymphodepleting preparative regimens used in the adoptive cell transfer protocols in the Surgery Branch, NCI.
Figure 2
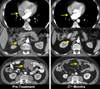
Objective clinical regressions in patients with metastatic melanoma treated with cell transfer therapy. A. Regression of melanoma metastases in the heart (upper), adrenal (middle) and peritoneal cavity (lower) now ongoing at 34 months in a 53 year old male. B. Regression of multiple liver metastases now ongoing at 60 months in a 45 year old male. C. Rapid regression of multiple subcutaneous and nodal metastases now ongoing at 35 months in a 29 year old male. D. Regression of a large fungating scalp mass now ongoing at 34 months in a 40 year old male.
Figure 2
Objective clinical regressions in patients with metastatic melanoma treated with cell transfer therapy. A. Regression of melanoma metastases in the heart (upper), adrenal (middle) and peritoneal cavity (lower) now ongoing at 34 months in a 53 year old male. B. Regression of multiple liver metastases now ongoing at 60 months in a 45 year old male. C. Rapid regression of multiple subcutaneous and nodal metastases now ongoing at 35 months in a 29 year old male. D. Regression of a large fungating scalp mass now ongoing at 34 months in a 40 year old male.
Figure 2
Objective clinical regressions in patients with metastatic melanoma treated with cell transfer therapy. A. Regression of melanoma metastases in the heart (upper), adrenal (middle) and peritoneal cavity (lower) now ongoing at 34 months in a 53 year old male. B. Regression of multiple liver metastases now ongoing at 60 months in a 45 year old male. C. Rapid regression of multiple subcutaneous and nodal metastases now ongoing at 35 months in a 29 year old male. D. Regression of a large fungating scalp mass now ongoing at 34 months in a 40 year old male.
Figure 2
Objective clinical regressions in patients with metastatic melanoma treated with cell transfer therapy. A. Regression of melanoma metastases in the heart (upper), adrenal (middle) and peritoneal cavity (lower) now ongoing at 34 months in a 53 year old male. B. Regression of multiple liver metastases now ongoing at 60 months in a 45 year old male. C. Rapid regression of multiple subcutaneous and nodal metastases now ongoing at 35 months in a 29 year old male. D. Regression of a large fungating scalp mass now ongoing at 34 months in a 40 year old male.
Figure 3
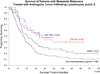
Survival of patients treated with cell transfer therapy in four consecutive clinical trials using increasing regimens of a lymphodepleting preparative regimen prior to adoptive cell transfer (NMA, non-myeloablative chemotherapy; TBI, total body irradiation). The number in parentheses is the number of patients in each trial.
Figure 4
Objective response rates using RECIST criteria in patients with metastatic melanoma treated in the Surgery Branch, NCI using different therapeutic strategies. Overall response rates in patients treated with vaccines is about 3% and with IL-2 or anti-CTLA4 is about 15%. With increasing levels of lymphodepletion, adding total body irradiation (TBI) and nonmyeloablative chemotherapy (NMA) to adoptive cell transfer (ACT) can achieve response rates as high as 72%.
Figure 5
Schematic diagrams of the structure of retroviruses encoding conventional T cell receptors and chimeric T cell receptors prepared for use in adoptive cell transfer trials.
Similar articles
- CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma.
Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, Restifo NP, Wunderlich JR, Prieto PA, Hong JJ, Langan RC, Zlott DA, Morton KE, White DE, Laurencot CM, Rosenberg SA. Dudley ME, et al. Clin Cancer Res. 2010 Dec 15;16(24):6122-31. doi: 10.1158/1078-0432.CCR-10-1297. Epub 2010 Jul 28. Clin Cancer Res. 2010. PMID: 20668005 Free PMC article. Clinical Trial. - Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function.
Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, Hwu P, Radvanyi LG. Hernandez-Chacon JA, et al. J Immunother. 2011 Apr;34(3):236-50. doi: 10.1097/CJI.0b013e318209e7ec. J Immunother. 2011. PMID: 21389874 Free PMC article. - Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes.
Friedman KM, Prieto PA, Devillier LE, Gross CA, Yang JC, Wunderlich JR, Rosenberg SA, Dudley ME. Friedman KM, et al. J Immunother. 2012 Jun;35(5):400-8. doi: 10.1097/CJI.0b013e31825898c5. J Immunother. 2012. PMID: 22576345 Free PMC article. - Adoptive T-cell transfer in melanoma.
Itzhaki O, Levy D, Zikich D, Treves AJ, Markel G, Schachter J, Besser MJ. Itzhaki O, et al. Immunotherapy. 2013 Jan;5(1):79-90. doi: 10.2217/imt.12.143. Immunotherapy. 2013. PMID: 23256800 Review. - Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care?
Svane IM, Verdegaal EM. Svane IM, et al. Cancer Immunol Immunother. 2014 Oct;63(10):1081-91. doi: 10.1007/s00262-014-1580-5. Epub 2014 Aug 7. Cancer Immunol Immunother. 2014. PMID: 25099366 Free PMC article. Review.
Cited by
- The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma.
Li J, Liu H, Li L, Wu H, Wang C, Yan Z, Wang Y, Su C, Jin H, Zhou F, Wu M, Qian Q. Li J, et al. Oncol Rep. 2013 Mar;29(3):895-902. doi: 10.3892/or.2012.2217. Epub 2012 Dec 28. Oncol Rep. 2013. PMID: 23292657 Free PMC article. - Harnessing DNA-induced immune responses for improving cancer vaccines.
Herrada AA, Rojas-Colonelli N, González-Figueroa P, Roco J, Oyarce C, Ligtenberg MA, Lladser A. Herrada AA, et al. Hum Vaccin Immunother. 2012 Nov 1;8(11):1682-93. doi: 10.4161/hv.22345. Epub 2012 Oct 30. Hum Vaccin Immunother. 2012. PMID: 23111166 Free PMC article. Review. - Tertiary lymphoid structure-related immune infiltrates in NSCLC tumor lesions correlate with low tumor-reactivity of TIL products.
Castenmiller SM, Kanagasabesan N, Guislain A, Nicolet BP, van Loenen MM, Monkhorst K, Veenhof AAFA, Smit EF, Hartemink KJ, Haanen JBAG, de Groot R, Wolkers MC. Castenmiller SM, et al. Oncoimmunology. 2024 Aug 22;13(1):2392898. doi: 10.1080/2162402X.2024.2392898. eCollection 2024. Oncoimmunology. 2024. PMID: 39188755 Free PMC article. - Transforming pancreaticobiliary cancer treatment: Exploring the frontiers of adoptive cell therapy and cancer vaccines.
Amhis N, Carignan J, Tai LH. Amhis N, et al. Mol Ther Oncol. 2024 Jun 8;32(3):200825. doi: 10.1016/j.omton.2024.200825. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39006944 Free PMC article. Review. - Adoptive T-cell therapy for malignant melanoma patients with TILs obtained by ultrasound-guided needle biopsy.
Ullenhag GJ, Sadeghi AM, Carlsson B, Ahlström H, Mosavi F, Wagenius G, Tötterman TH. Ullenhag GJ, et al. Cancer Immunol Immunother. 2012 May;61(5):725-32. doi: 10.1007/s00262-011-1182-4. Epub 2011 Dec 23. Cancer Immunol Immunother. 2012. PMID: 22202906 Free PMC article.
References
- Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol. 1987;138:989–995. - PubMed
- Van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. - PubMed
- Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. - PubMed
- Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. Three lymphodepleting regimens were combined with tumor reactive TIL administration and compared for safety and efficacy in sequential ACT clinical trials. Overall 52 of 93 patients (56%) experienced an objective response
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials