Phosphorylation of the SRC epithelial substrate Trask is tightly regulated in normal epithelia but widespread in many human epithelial cancers - PubMed (original) (raw)
Phosphorylation of the SRC epithelial substrate Trask is tightly regulated in normal epithelia but widespread in many human epithelial cancers
Ching Hang Wong et al. Clin Cancer Res. 2009.
Abstract
Purpose: The frequently elevated activities of the c-src and c-yes products in human epithelial tumors suggest that these activated tyrosine kinases have tumorigenic functions analogous to the v-src and v-yes oncogene products. Studies of v-src-transformed fibroblasts have identified many of the effectors of this potent oncogene; however, because c-src and c-yes lack the mutational and promiscuous activities of their retroviral oncogene homologues, their presumptive tumorigenic functions in human epithelial tumors are more subtle, less well-defined, and await identification of possible effectors more directly relevant to epithelial cells.
Experimental design: We recently identified a transmembrane glycoprotein named Trask that is expressed in epithelial tissues but not fibroblasts and is phosphorylated by SRC kinases in mitotic epithelial cells. In this study, we have surveyed the expression and phosphorylation of Trask in many human epithelial cancer cell lines and surgical tissues and tumors.
Results: Trask is widely expressed in human epithelial tissues, but its phosphorylation is tightly regulated and restricted to detached mitotic cells or cells undergoing physiologic shedding. However, abberant Trask phosphorylation is seen in many epithelial tumors from all stages including preinvasive, invasive, and metastatic tumors. Trask phosphorylation requires SRC kinases, and is also aberrantly hyperphosphorylated in the SRC-activated PyMT mouse epithelial tumors and dephosphorylated by the SRC inhibitor treatment of these tumors.
Conclusions: The widespread phosphorylation of Trask in many human epithlelial cancers identifies a new potential effector of SRC kinases in human epithelial tumorigenesis.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
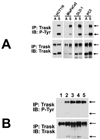
Figure 1
The expression and phosphorylation of Trask were determined and compared in a diverse panel of epithelial cancer cell lines. Total cell lysates were immunoprecipitated with rabbit anti-Trask antibodies and immunoblotted with mouse anti-Trask antibodies or anti-phosphotyrosine antibodies. The MDA-231 cell lysate was included in all blots as a basis to allow comparison of Trask expression and phosphorylation across different blots. The MCF10A cell lysate is also included at the far right as an example of untransformed epithelial cells. Arrows indicate the 140kd and 85kd forms of Trask.
Figure 2
A) The HCT116 and DLD-1 (colon cancer), MiaPaCa2 (pancreatic cancer), and PC3 (prostate cancer) epithelial cancer cells were harvested either while adherent (labelled A) or 2 hours after being forced into suspension by EDTA (labelled S). Cell lysates were analyzed for the expression and phosphorylation of Trask as indicated. Arrows indicate the 140kd and 85kd forms of Trask. B) The expression and phosphorylation of Trask was determined and compared in L3.6pl-luc pancreatic cancer cells under different in vitro and in vivo circumstances. These L3.6pl-luc cells express luciferase for use in in vivo imaging. All immunoprecipitates are from equal amounts of cell lysate. Lane 1 and 2 correspond to lysates from adherent and suspended cells respectively, growing in vitro. These cells were also grown orthotopically within the pancreas of nude mice and their growth monitored by in vivo imaging as described in methods. Well established pancreatic tumors from two different sacrificed mice were harvested, snap frozen, and their lysates used in lanes 3,4. A peritoneal metastasis from one of the mice was removed and lysate used in lane 5. These data comparing the same cancer cells in vitro and in vivo show that despite the more restricted phosphorylation of Trask in the in vitro growth model, their tumorigenic growth in vivo is characterized by maximal and constitutive phosphorylation of Trask.
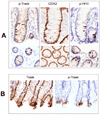
Figure 3
A) Sections from normal colon biopsies were prepared and stained with anti-phospho-Trask antibodies. The staining intensity was scored by two observers according to procedures and definitions described in methods. All immunostains and their analyses were conducted using well defined positive and negative controls as described in methods. Trask phosphorylation is seen in occasional mitotic cells. This is shown here in the mitotically active crypt regions of normal colonic mucosa. The detaching cells in the crypts were confirmed to be epithelial cells and not extrinsic cells as shown by staining with the intestinal epithelial differentiation marker CDX2 and confirmed to be mitotic cells by the mitotic marker phospho-histone H3. B) Trask phosphorylation is also seen in some clusters of cells at the apices of colonic villi where shedding commonly occurs.
Figure 4
A) Representative images from immunostains of colon tubular adenomas is shown. In contrast to normal colonic crypts, where Trask phosphorylation is restricted to mitotic cells, in adenomas there is abundant Trask phosphorylation in many clusters and foci of tumor cells. There is also abundant tumor cell luminal shedding. B) The frequent luminal shedding in adnomas is non-mitotic as confirmed by the absence of phospho-Histone H3 staining.
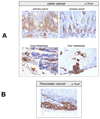
Figure 5
Representative images from phospho-Trask immunostains are shown for a number of subtypes of epithelial cancers as indicated.
Figure 5
Representative images from phospho-Trask immunostains are shown for a number of subtypes of epithelial cancers as indicated.
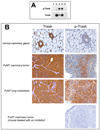
Figure 6
A) SYF cells were transfected with a pcDNA4-MycTrask expression vector and either Src or Yes expressing vectors and the expressed Trask protein was immunoprecipitated with anti-myc antibodies and immunoblotted with anti-phosphotyrosine or anti-Trask antibodies. Lanes correspond to 1) untransfected cells, 2) cells transfected with pcDNA4-MycTrask and pCMV6 vector, 3) cells transfected with pcDNA4-MycTrask and pCMV6-src, 4) cells transfected with pcDNA4-MycTrask and pCMV6-yes. Lane 5 contains the same lysate as lane 4 but was immunoprecipitated with IgG control. B) PyMT induced mammary tumors were generated in mice as described in Methods and allowed to grow to about 2.5 cm in size. Mice were sacrificied and tumor tissues were fixed in formalin and lung metastases were dissected, identified and fixed. Sections of normal mammary tissue from control mice as well as sections from PyMT induced tumors and their lung metastases were studied by immunohistochemical analyses to determine the expression and phosphorylation of Trask. Tumors from mice treated with the Src inhibitor dasatinib for 1 week were also harvested for analysis. Trask phosphorylation is induced in this tumor model and dephosphorylated by Src inhibitor therapy. C) Mice were orthotopically implanted with PyMT induced syngeneic tumors and after tumors were established at approximately one month, 30 tumor-bearing mice were randomized to two arms and treated with dasatinib or DMSO control and tumor sizes were measured twice weekly.
Figure 6
A) SYF cells were transfected with a pcDNA4-MycTrask expression vector and either Src or Yes expressing vectors and the expressed Trask protein was immunoprecipitated with anti-myc antibodies and immunoblotted with anti-phosphotyrosine or anti-Trask antibodies. Lanes correspond to 1) untransfected cells, 2) cells transfected with pcDNA4-MycTrask and pCMV6 vector, 3) cells transfected with pcDNA4-MycTrask and pCMV6-src, 4) cells transfected with pcDNA4-MycTrask and pCMV6-yes. Lane 5 contains the same lysate as lane 4 but was immunoprecipitated with IgG control. B) PyMT induced mammary tumors were generated in mice as described in Methods and allowed to grow to about 2.5 cm in size. Mice were sacrificied and tumor tissues were fixed in formalin and lung metastases were dissected, identified and fixed. Sections of normal mammary tissue from control mice as well as sections from PyMT induced tumors and their lung metastases were studied by immunohistochemical analyses to determine the expression and phosphorylation of Trask. Tumors from mice treated with the Src inhibitor dasatinib for 1 week were also harvested for analysis. Trask phosphorylation is induced in this tumor model and dephosphorylated by Src inhibitor therapy. C) Mice were orthotopically implanted with PyMT induced syngeneic tumors and after tumors were established at approximately one month, 30 tumor-bearing mice were randomized to two arms and treated with dasatinib or DMSO control and tumor sizes were measured twice weekly.
Similar articles
- The transmembrane src substrate Trask is an epithelial protein that signals during anchorage deprivation.
Spassov DS, Baehner FL, Wong CH, McDonough S, Moasser MM. Spassov DS, et al. Am J Pathol. 2009 May;174(5):1756-65. doi: 10.2353/ajpath.2009.080890. Epub 2009 Apr 6. Am J Pathol. 2009. PMID: 19349359 Free PMC article. - Trask phosphorylation defines the reverse mode of a phosphotyrosine signaling switch that underlies cell anchorage state.
Spassov DS, Wong CH, Moasser MM. Spassov DS, et al. Cell Cycle. 2011 Apr 15;10(8):1225-32. doi: 10.4161/cc.10.8.15343. Epub 2011 Apr 15. Cell Cycle. 2011. PMID: 21490433 Free PMC article. - Phosphorylation of Trask by Src kinases inhibits integrin clustering and functions in exclusion with focal adhesion signaling.
Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, Moasser MM. Spassov DS, et al. Mol Cell Biol. 2011 Feb;31(4):766-82. doi: 10.1128/MCB.00841-10. Epub 2010 Dec 28. Mol Cell Biol. 2011. PMID: 21189288 Free PMC article. - Src family kinases in tumor progression and metastasis.
Summy JM, Gallick GE. Summy JM, et al. Cancer Metastasis Rev. 2003 Dec;22(4):337-58. doi: 10.1023/a:1023772912750. Cancer Metastasis Rev. 2003. PMID: 12884910 Review. - The cell surface glycoprotein CDCP1 in cancer--insights, opportunities, and challenges.
Wortmann A, He Y, Deryugina EI, Quigley JP, Hooper JD. Wortmann A, et al. IUBMB Life. 2009 Jul;61(7):723-30. doi: 10.1002/iub.198. IUBMB Life. 2009. PMID: 19514048 Review.
Cited by
- An oncogenic viral interferon regulatory factor upregulates CUB domain-containing protein 1 to promote angiogenesis by hijacking transcription factor lymphoid enhancer-binding factor 1 and metastasis suppressor CD82.
Li W, Wang Q, Qi X, Lu H, Chen Y, Shi J, Wang F, Wang Z, Lu Y, Lu Z, Yan Q, Wang C, Gao SJ, Lu C. Li W, et al. Cell Death Differ. 2020 Dec;27(12):3289-3306. doi: 10.1038/s41418-020-0578-0. Epub 2020 Jun 17. Cell Death Differ. 2020. PMID: 32555380 Free PMC article. - Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer.
Arcaroli JJ, Touban BM, Tan AC, Varella-Garcia M, Powell RW, Eckhardt SG, Elvin P, Gao D, Messersmith WA. Arcaroli JJ, et al. Clin Cancer Res. 2010 Aug 15;16(16):4165-77. doi: 10.1158/1078-0432.CCR-10-0066. Epub 2010 Aug 3. Clin Cancer Res. 2010. PMID: 20682712 Free PMC article. - P2X(7) receptor antagonists display agonist-like effects on cell signaling proteins.
Hedden L, Benes CH, Soltoff SP. Hedden L, et al. Biochim Biophys Acta. 2011 May;1810(5):532-42. doi: 10.1016/j.bbagen.2011.03.009. Epub 2011 Mar 21. Biochim Biophys Acta. 2011. PMID: 21397667 Free PMC article. - Significance of Trask protein interactions in brain metastatic cohorts of lung cancers.
Wu H, Shang LQ, Chen RL, Yang SM, Wang SL, Wang J, Sun G. Wu H, et al. Tumour Biol. 2015 Jun;36(6):4181-7. doi: 10.1007/s13277-015-3053-7. Epub 2015 Jan 21. Tumour Biol. 2015. PMID: 25775948 - SRC kinase-mediated signaling pathways and targeted therapies in breast cancer.
Luo J, Zou H, Guo Y, Tong T, Ye L, Zhu C, Deng L, Wang B, Pan Y, Li P. Luo J, et al. Breast Cancer Res. 2022 Dec 29;24(1):99. doi: 10.1186/s13058-022-01596-y. Breast Cancer Res. 2022. PMID: 36581908 Free PMC article. Review.
References
- Martin GS. The road to Src. Oncogene. 2004;23:7910–7917. - PubMed
- Cartwright CA, Eckhart W, Simon S, Kaplan PL. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987;49:83–91. - PubMed
- Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. - PubMed
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. - PubMed
- Muthuswamy SK, Muller WJ. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene. 1995;11:1801–1810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA113952-04/CA/NCI NIH HHS/United States
- CA113952/CA/NCI NIH HHS/United States
- R01 CA113952-03/CA/NCI NIH HHS/United States
- R01 CA113952-02/CA/NCI NIH HHS/United States
- R01 CA113952/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous