Molecular neuropathology of gliomas - PubMed (original) (raw)
Review
Molecular neuropathology of gliomas
Markus J Riemenschneider et al. Int J Mol Sci. 2009 Jan.
Abstract
Gliomas are the most common primary human brain tumors. They comprise a heterogeneous group of benign and malignant neoplasms that are histologically classified according to the World Health Organization (WHO) classification of tumors of the nervous system. Over the past 20 years the cytogenetic and molecular genetic alterations associated with glioma formation and progression have been intensely studied and genetic profiles as additional aids to the definition of brain tumors have been incorporated in the WHO classification. In fact, first steps have been undertaken in supplementing classical histopathological diagnosis by the use of molecular tests, such as MGMT promoter hypermethylation in glioblastomas or detection of losses of chromosome arms 1p and 19q in oligodendroglial tumors. The tremendous progress that has been made in the use of array-based profiling techniques will likely contribute to a further molecular refinement of glioma classification and lead to the identification of glioma core pathways that can be specifically targeted by more individualized glioma therapies.
Keywords: 19q; 1p; Glioblastoma; MGMT; biomarker; ependymoma; genetics; molecular diagnostics; oligodendroglioma; profiling.
Figures
Figure 1.
Schematic representation of the molecular pathogenesis and progression of diffusely infiltrating astrocytic gliomas.
Figure 2.
Schematic representation of the molecular pathogenesis of pilocytic astrocytomas.
Figure 3.
Schematic representation of the molecular pathogenesis and progression of oligodendrogliomas.
Figure 4.
Schematic representation of the molecular pathogenesis and progression of oligoastrocytomas.
Figure 5.
Schematic representation of the molecular pathogenesis and progression of ependymal gliomas.
Figure 6.
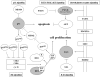
Core pathways involved in the pathogenesis of gliomas. Note the interrelationships between p53, RB, growth factor receptor, PTEN/PI3K/AKT and RAS signaling on the regulation of cell proliferation and apoptosis. While TP53 mutation, amplification of MDM2/MDM4 or p14 ARF deletion/methylation inhibits apoptosis, alterations in p16INK4a, p15INK4b, p18INK4c and p21waf1 disinhibit cell cycle progression at the G1/S-phase checkpoint via cyclin-dependent kinases by phosphorylation of RB1 and release of E2F transcription factors. Amplification, overexpression or mutation of growth factor receptors stimulates cell proliferation and inhibits apoptosis through both the RAS as well as the PI3K/AKT signaling pathway. The RAS signaling pathway can be alternatively activated by mutations in the NF1, the PI3K/AKT signaling pathway by mutations in the PTEN gene and less commonly, the PIK3CA or the PIK3R1 gene.
Similar articles
- Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation.
Brito C, Azevedo A, Esteves S, Marques AR, Martins C, Costa I, Mafra M, Bravo Marques JM, Roque L, Pojo M. Brito C, et al. BMC Cancer. 2019 Oct 17;19(1):968. doi: 10.1186/s12885-019-6177-0. BMC Cancer. 2019. PMID: 31623593 Free PMC article. - Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice.
Weller M, Stupp R, Hegi ME, van den Bent M, Tonn JC, Sanson M, Wick W, Reifenberger G. Weller M, et al. Neuro Oncol. 2012 Sep;14 Suppl 4(Suppl 4):iv100-8. doi: 10.1093/neuonc/nos206. Neuro Oncol. 2012. PMID: 23095825 Free PMC article. Review. - Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial.
Wiestler B, Capper D, Hovestadt V, Sill M, Jones DT, Hartmann C, Felsberg J, Platten M, Feiden W, Keyvani K, Pfister SM, Wiestler OD, Meyermann R, Reifenberger G, Pietsch T, von Deimling A, Weller M, Wick W. Wiestler B, et al. Neuro Oncol. 2014 Dec;16(12):1630-8. doi: 10.1093/neuonc/nou138. Epub 2014 Jul 15. Neuro Oncol. 2014. PMID: 25028501 Free PMC article. - Correlation of chromosomes 1p and 19q status and expressions of O6-methylguanine DNA methyltransferase (MGMT), p53 and Ki-67 in diffuse gliomas of World Health Organization (WHO) grades II and III: a clinicopathological study.
Huang L, Jiang T, Yuan F, Li GL, Cui Y, Liu EZ, Wang ZC. Huang L, et al. Neuropathol Appl Neurobiol. 2009 Aug;35(4):367-379. doi: 10.1111/j.1365-2990.2008.01002.x. Epub 2008 Nov 18. Neuropathol Appl Neurobiol. 2009. PMID: 19019173 - Clinical impact of molecular biomarkers in gliomas.
Siegal T. Siegal T. J Clin Neurosci. 2015 Mar;22(3):437-44. doi: 10.1016/j.jocn.2014.10.004. Epub 2014 Dec 18. J Clin Neurosci. 2015. PMID: 25533211 Review.
Cited by
- Human Cytomegalovirus DNA Quantification and Gene Expression in Gliomas of Different Grades.
Stangherlin LM, Castro FL, Medeiros RS, Guerra JM, Kimura LM, Shirata NK, Nonogaki S, Dos Santos CJ, Carlan Silva MC. Stangherlin LM, et al. PLoS One. 2016 Jul 26;11(7):e0159604. doi: 10.1371/journal.pone.0159604. eCollection 2016. PLoS One. 2016. PMID: 27458810 Free PMC article. - Role of tenascins in the ECM of gliomas.
Brösicke N, Faissner A. Brösicke N, et al. Cell Adh Migr. 2015;9(1-2):131-40. doi: 10.1080/19336918.2014.1000071. Cell Adh Migr. 2015. PMID: 25695402 Free PMC article. Review. - Ibrutinib, a Bruton's tyrosine kinase inhibitor, exhibits antitumoral activity and induces autophagy in glioblastoma.
Wang J, Liu X, Hong Y, Wang S, Chen P, Gu A, Guo X, Zhao P. Wang J, et al. J Exp Clin Cancer Res. 2017 Jul 17;36(1):96. doi: 10.1186/s13046-017-0549-6. J Exp Clin Cancer Res. 2017. PMID: 28716053 Free PMC article. - MiR-328 promotes glioma cell invasion via SFRP1-dependent Wnt-signaling activation.
Delic S, Lottmann N, Stelzl A, Liesenberg F, Wolter M, Götze S, Zapatka M, Shiio Y, Sabel MC, Felsberg J, Reifenberger G, Riemenschneider MJ. Delic S, et al. Neuro Oncol. 2014 Jan;16(2):179-90. doi: 10.1093/neuonc/not164. Epub 2013 Dec 4. Neuro Oncol. 2014. PMID: 24305703 Free PMC article. - The Na, K-ATPase β-Subunit Isoforms Expression in Glioblastoma Multiforme: Moonlighting Roles.
Rotoli D, Cejas MM, Maeso MD, Pérez-Rodríguez ND, Morales M, Ávila J, Mobasheri A, Martín-Vasallo P. Rotoli D, et al. Int J Mol Sci. 2017 Nov 8;18(11):2369. doi: 10.3390/ijms18112369. Int J Mol Sci. 2017. PMID: 29117147 Free PMC article.
References
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. (Berl.) 2005;109:93–108. - PubMed
- Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J. Clin. Oncol. 2006;24:2707–2714. - PubMed
- van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MC, Vecht CJ, Allgeier A, Lacombe D, Gorlia T. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J. Clin. Oncol. 2006;24:2715–2722. - PubMed
- Nishizaki T, Ozaki S, Harada K, Ito H, Arai H, Beppu T, Sasaki K. Investigation of genetic alterations associated with the grade of astrocytic tumor by comparative genomic hybridization. Genes Chromosom. Cancer. 1998;21:340–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous