Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy - PubMed (original) (raw)
Review
Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy
Guojun Bu. Nat Rev Neurosci. 2009 May.
Abstract
The vast majority of Alzheimer's disease (AD) cases are late-onset and their development is probably influenced by both genetic and environmental risk factors. A strong genetic risk factor for late-onset AD is the presence of the epsilon4 allele of the apolipoprotein E (APOE) gene, which encodes a protein with crucial roles in cholesterol metabolism. There is mounting evidence that APOE4 contributes to AD pathogenesis by modulating the metabolism and aggregation of amyloid-beta peptide and by directly regulating brain lipid metabolism and synaptic functions through APOE receptors. Emerging knowledge of the contribution of APOE to the pathophysiology of AD presents new opportunities for AD therapy.
Figures
Figure 1. Schematic representation of human apoE
The 299-amino acid human apoE contains two independently folded domains: an N-terminal domain that includes the receptor-binding region and a C-terminal domain that contains the major lipid-binding region. The residues that distinguish the apoE isoforms (112 and 158) are marked. ApoE2 has cysteines at both positions, apoE4 has arginines at both positions, and apoE3 has Cys at position 112 and Arg at position 158. Domain interaction between Arg61 and Glu255 in apoE4 is also indicated.
Figure 2. ApoE receptors, members of the LDLR family
Structural organization of the LDLR family members. All receptors are type I receptors, each containing a single membrane-spanning domain and a relatively short cytoplasmic tail. The extracellular regions of these receptors contain three characteristic modules: ligand-binding repeats (also called complement-type repeats), EGF repeats and YWTD-containing β-propeller domains. The furin cleavage sites in LRP1 and LRP1B are indicated with purple arrow heads. The four clusters of ligand-binding repeats in LRP1 are labelled (I-IV). Highlighted in bright green are the two extra sequences in LRP1B encoded by two extra exons (compared to LRP1): a ligand-binding repeat in the fourth ligand-binding domain and a 33-amino acid insert in the cytoplasmic tail. LRP5/6 and sorLA/LR11 are distant members of the family with atypical structural arrangements. Several other LDLR family members, including LRP3, LRP9 and LRP12, whose functions are poorly defined, are not depicted here.
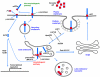
Figure 3. APP processing pathways regulated by LDLR family members and apoE
Newly synthesized APP traffics through the secretory pathway to the plasma membrane, where it is primarily cleaved by the α-secretase in the non-amyloidogenic pathway, producing a minimally toxic peptide P3. When APP is internalized through the clathrin-mediated pathway, it is delivered to endosomes, where it is cleaved first by the β-secretase (BACE1), and then by the γ-secretase to generate highly toxic Aβ. Although most Aβ peptides are secreted to extracellular space, some Aβ peptides may aggregate in the late endosomes/lysosomes contributing to intraneuronal Aβ accumulation. The fast endocytosis of LRP1 enhances APP endocytosis and processing to Aβ, whereas the slow endocytosis of LRP1B and apoER2 contributes to retaining APP at the cell surface and promotes non-amyloidogenic processing. SorLA/LR11 likely shuttles APP to the Golgi compartments and reduces its processing by β-secretase in the early endosomes, thus decreasing Aβ production. ApoE4 promotes APP amyloidogenic processing in a manner that depends on LRP1 function. APP’s intracellular domain (AICD), a product of both non-amyloidgenic and amyloidgenic processing, interacts with FE65 and Tip60, and regulates gene transcription in the nucleus.
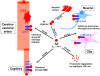
Figure 4. Major Aβ clearance pathways in the brain: role of apoE isoforms
Aβ accumulation in the brain parenchyma leads to formation of Aβ oligomers and amyloid plaques, which are toxic to neurons, whereas its accumulation in the perivascular region leads to the formation of CAA, which disrupts vessel function and is associated with cerebral haemorrhage. Aβ has a relatively short half-life in the brain, ~4 h in older mice and ~6 h in humans. Major Aβ clearance pathways include receptor-mediated clearance by cells in brain parenchyma (by neurons and glia), along the interstitial fluid (ISF) drainage pathway, through the blood-brain barrier (BBB) and proteolytic degradation by endopeptidases. The comparative effects of apoE3 and apoE4 are indicated. In all major cellular Aβ clearance pathways, LRP1 is involved and is likely to clear Aβ either directly or when Aβ binds to its chaperones that are also LRP1 ligands (e.g. apoE, α2M). LDLR function in Aβ clearance is likely to involve Aβ-apoE complexes. VLDLR also has a role in the clearance of Aβ-apoE complexes at the BBB.
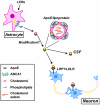
Figure 5. Synapse formation and repair depend on cholesterol transport from astrocytes to neurons via the apoE/apoE receptor pathway
ApoE, secreted by astrocytes, assembles cholesterol and other lipids into lipoprotein particles. The ATP-binding cassette transporter subfamily A member 1 (ABCA1) at the plasma membrane transports and loads lipids to apoE. Liver X receptors (LXRs) increase the expression of both ABCA1 and apoE. ApoE/lipoprotein particles may undergo modifications such as recruiting oligodendrocyte-specific lipids and additional apoE molecules prior to binding to neuronal apoE receptors LRP1/LDLR or being transported to the cerebrospinal fluid (CSF). Cholesterol and other lipids transported to neurons play important roles in synaptic formation and repair (see main text for details). The inset shows the main components of apoE/lipoprotein particles: cholesterol, cholesterol esters and phospholipids.
Figure 6. Roles of apoE isoforms in the healthy brain and AD pathogenesis
Summary of apoE functions in normal brain function and the pathogenic processes of AD. Differential regulations by apoE3 and apoE4 are indicated.
References
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 2003;26:565–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AG027924/AG/NIA NIH HHS/United States
- R01AG031784/AG/NIA NIH HHS/United States
- R01 AG031784/AG/NIA NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous