Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress - PubMed (original) (raw)
Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress
R L Hull et al. Diabetologia. 2009 Jun.
Abstract
Aims/hypothesis: Supraphysiological levels of the amyloidogenic peptide human islet amyloid polypeptide have been associated with beta cell endoplasmic reticulum (ER) stress. However, in human type 2 diabetes, levels of human IAPP are equivalent or decreased relative to matched controls. Thus, we sought to investigate whether ER stress is induced during amyloidogenesis at physiological levels of human IAPP.
Methods: Islets from human IAPP transgenic mice that develop amyloid, and non-transgenic mice that do not, were cultured for up to 7 days in 11.1, 16.7 and 33.3 mmol/l glucose. Pancreases from human IAPP transgenic and non-transgenic mice and humans with or without type 2 diabetes were also evaluated. Amyloid formation was determined histologically. ER stress was determined in islets by quantifying mRNA levels of Bip, Atf4 and Chop (also known as Ddit3) and alternate splicing of Xbp1 mRNA, or in pancreases by immunostaining for immunoglobulin heavy chain-binding protein (BIP), C/EBP homologous protein (CHOP) and X-box binding protein 1 (XBP1).
Results: Amyloid formation in human IAPP transgenic islets was associated with reduced beta cell area in a glucose- and time-dependent manner. However, amyloid formation was not associated with significant increases in expression of ER stress markers under any culture condition. Thapsigargin treatment, a positive control, did result in significant ER stress. Amyloid formation in vivo in pancreas samples from human IAPP transgenic mice or humans was not associated with upregulation of ER stress markers.
Conclusions/interpretation: Our data suggest that ER stress is not an obligatory pathway mediating the toxic effects of amyloid formation at physiological levels of human IAPP.
Figures
Figure 1
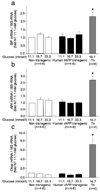
mRNA levels by real time PCR for BiP (panel a), Atf4 (panel b) and Chop (panel c), in non-transgenic (open bars) or hIAPP transgenic mouse islets (solid bars) cultured for 7 days in 11.1, 16.7 or 33.3 mmol/l glucose or in non-transgenic islets 16.7 mmol/l glucose + 0.3 µM thapsigargin (grey bars) for the final four hours (n=4–6 per condition). While thapsigargin treatment led to marked increases in mRNA levels of all three ER stress markers, there were no significant differences in BiP, Atf4 or Chop mRNA levels among non-transgenic or hIAPP transgenic islets at any glucose concentration.
Figure 2
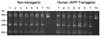
Xbp-1 mRNA splicing products analyzed by agarose gel electrophoresis in non-transgenic (lanes 1–3) or hIAPP transgenic mouse islets (lanes 4–6) cultured for seven days in 11.1 (lanes 1 and 4), 16.7 (lanes 2 and 5) or 33.3 mmol/l glucose (lanes 3 and 6) or non-transgenic islets cultured for seven days in 16.7 mmol/l glucose + 0.3 µM thapsigargin for the final four hours (Th). The four bands on the gel are the PCR products from intact (473 bp) normally spliced Xbp-1 mRNA and its two digestion products produced from PstI restriction digestion (product 1 at 290 bp and product 2 at 183 bp) and the alternatively spliced form of Xbp-1 mRNA (447 bp). While thapsigargin treatment led to almost complete generation of the alternatively spliced Xbp-1 mRNA, there were no differences in the proportion of the four bands in any of the experimental conditions, suggesting that there were no differences in Xbp-1 mRNA splicing among non-transgenic or hIAPP transgenic islets at any glucose concentration.
Figure 3
mRNA levels by real time PCR for BiP (panel a and b), Atf4 (panel c and d) and Chop (panel e and f), in non-transgenic (open bars; panels a, c and e) or hIAPP transgenic mouse islets (solid bars; panels b, d and f) cultured for up to 7 days in 16.7 mmol/l glucose. Atf4 and Chop mRNA levels decreased between day 1 and day 2 in both genotypes, but there were no significant differences in BiP, Atf4 or Chop mRNA levels between non-transgenic or hIAPP transgenic islets at any time point (n=3 per condition).
Figure 4
Xbp-1 mRNA splicing products analyzed by agarose gel electrophoresis as described in Figure 2 in non-transgenic or hIAPP transgenic mouse islets (lanes 1–7; days 1–7 respectively) cultured for up to seven days in 16.7 mmol/l glucose or in 16.7 mmol/l glucose + 0.3 µM thapsigargin for the final four hours (Th). While thapsigargin treatment led to almost complete generation of the alternatively spliced Xbp-1 mRNA, there were no differences in Xbp-1 mRNA splicing between non-transgenic or hIAPP transgenic islets at any time point.
Figure 5
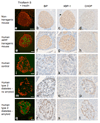
Pancreas sections from high fat fed non-transgenic (panels a-d) and hIAPP transgenic (panels e–h) mice and from human subjects without diabetes (panels i–l), with type 2 diabetes but without amyloid deposition (panels m–p), and with type 2 diabetes and significant amyloid deposition (panels q–t). Pancreas sections were stained for insulin + thioflavin S (panels a, e, i, m, q), BiP (panels b, f, j, n, r), XBP-1 (panels c, g, k, o, s) or CHOP (panels d, h, l, p, t). Amyloid deposition and decreased beta cell area were evident in amyloid-containing pancreata from the hIAPP transgenic mouse and the human subject with type 2 diabetes (panels e and q). BiP and XBP-1 immunostaining were present to an equivalent degree in islets from both non-transgenic and hIAPP transgenic mice, whereas CHOP immunostaining was not observed in mouse pancreas. Note that the faint nuclear staining in non-transgenic mouse pancreas (panel d) is deemed to be non-specific staining as it is present to a similar degree throughout the islet and exocrine pancreas. BiP and XBP-1 staining were absent from human islets, whereas cytoplasmic staining for CHOP was present in islets from subjects with type 2 diabetes, regardless of the presence of amyloid.
Similar articles
- The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients.
Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. Marchetti P, et al. Diabetologia. 2007 Dec;50(12):2486-94. doi: 10.1007/s00125-007-0816-8. Epub 2007 Sep 30. Diabetologia. 2007. PMID: 17906960 - Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide.
Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Huang CJ, et al. Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1656-62. doi: 10.1152/ajpendo.00318.2007. Epub 2007 Oct 2. Am J Physiol Endocrinol Metab. 2007. PMID: 17911343 - Exendin-4 increases islet amyloid deposition but offsets the resultant beta cell toxicity in human islet amyloid polypeptide transgenic mouse islets.
Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Aston-Mourney K, et al. Diabetologia. 2011 Jul;54(7):1756-65. doi: 10.1007/s00125-011-2143-3. Epub 2011 Apr 12. Diabetologia. 2011. PMID: 21484213 Free PMC article. - Islet amyloid polypeptide, islet amyloid, and diabetes mellitus.
Westermark P, Andersson A, Westermark GT. Westermark P, et al. Physiol Rev. 2011 Jul;91(3):795-826. doi: 10.1152/physrev.00042.2009. Physiol Rev. 2011. PMID: 21742788 Review. - [Construction of transgenic mouse system expressing human islet amyloid polypeptide (IAPP)/amylin].
Yagui K, Kanatsuka A, Makino H. Yagui K, et al. Nihon Rinsho. 1994 Oct;52(10):2746-50. Nihon Rinsho. 1994. PMID: 7983808 Review. Japanese.
Cited by
- Beta-cell deterioration during diabetes: what's in the gun?
Robertson RP. Robertson RP. Trends Endocrinol Metab. 2009 Oct;20(8):388-93. doi: 10.1016/j.tem.2009.05.004. Epub 2009 Sep 11. Trends Endocrinol Metab. 2009. PMID: 19748794 Free PMC article. Review. - Mechanisms of islet amyloidosis toxicity in type 2 diabetes.
Abedini A, Schmidt AM. Abedini A, et al. FEBS Lett. 2013 Apr 17;587(8):1119-27. doi: 10.1016/j.febslet.2013.01.017. Epub 2013 Jan 18. FEBS Lett. 2013. PMID: 23337872 Free PMC article. Review. - Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies.
Costes S, Bertrand G, Ravier MA. Costes S, et al. Int J Mol Sci. 2021 May 18;22(10):5303. doi: 10.3390/ijms22105303. Int J Mol Sci. 2021. PMID: 34069914 Free PMC article. Review. - Potential Effects of Hyperglycemia on SARS-CoV-2 Entry Mechanisms in Pancreatic Beta Cells.
Michaels TM, Essop MF, Joseph DE. Michaels TM, et al. Viruses. 2024 Aug 2;16(8):1243. doi: 10.3390/v16081243. Viruses. 2024. PMID: 39205219 Free PMC article. Review. - Islet amyloid: from fundamental biophysics to mechanisms of cytotoxicity.
Cao P, Marek P, Noor H, Patsalo V, Tu LH, Wang H, Abedini A, Raleigh DP. Cao P, et al. FEBS Lett. 2013 Apr 17;587(8):1106-18. doi: 10.1016/j.febslet.2013.01.046. Epub 2013 Feb 1. FEBS Lett. 2013. PMID: 23380070 Free PMC article. Review.
References
- Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77:91–94. - PubMed
- Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis - quantitative changes in the pancreas in type-2 diabetes. Diab Res Clin Exptl. 1988;9:151–159. - PubMed
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type 2 diabetes mellitus. Nature. 1994;368:756–760. - PubMed
- Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. - PubMed
- Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007247/DK/NIDDK NIH HHS/United States
- P30 DK017047-33/DK/NIDDK NIH HHS/United States
- DK07247/DK/NIDDK NIH HHS/United States
- R01 DK075998-01A2/DK/NIDDK NIH HHS/United States
- K99 DK080945-01A1/DK/NIDDK NIH HHS/United States
- DK17047/DK/NIDDK NIH HHS/United States
- K01 DK074404-03/DK/NIDDK NIH HHS/United States
- T32 DK007247-32/DK/NIDDK NIH HHS/United States
- DK74404/DK/NIDDK NIH HHS/United States
- DK75998/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- K01 DK074404/DK/NIDDK NIH HHS/United States
- R01 DK075998/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials