Inhibition of prostaglandin D synthase suppresses muscular necrosis - PubMed (original) (raw)
Inhibition of prostaglandin D synthase suppresses muscular necrosis
Ikuko Mohri et al. Am J Pathol. 2009 May.
Abstract
Duchenne muscular dystrophy is a fatal muscle wasting disease that is characterized by a deficiency in the protein dystrophin. Previously, we reported that the expression of hematopoietic prostaglandin D synthase (HPGDS) appeared in necrotic muscle fibers from patients with either Duchenne muscular dystrophy or polymyositis. HPGDS is responsible for the production of the inflammatory mediator, prostaglandin D(2). In this paper, we validated the hypothesis that HPGDS has a role in the etiology of muscular necrosis. We investigated the expression of HPGDS/ prostaglandin D(2) signaling using two different mouse models of muscle necrosis, that is, bupivacaine-induced muscle necrosis and the mdx mouse, which has a genetic muscular dystrophy. We treated each mouse model with the HPGDS-specific inhibitor, HQL-79, and measured both necrotic muscle volume and selected cytokine mRNA levels. We confirmed that HPGDS expression was induced in necrotic muscle fibers in both bupivacaine-injected muscle and mdx mice. After administration of HQL-79, necrotic muscle volume was significantly decreased in both mouse models. Additionally, mRNA levels of both CD11b and transforming growth factor beta1 were significantly lower in HQL-79-treated mdx mice than in vehicle-treated animals. We also demonstrated that HQL-79 suppressed prostaglandin D(2) production and improved muscle strength in the mdx mouse. Our results show that HPGDS augments inflammation, which is followed by muscle injury. Furthermore, the inhibition of HPGDS ameliorates muscle necrosis even in cases of genetic muscular dystrophy.
Figures
Figure 1
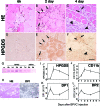
A–H: HPGDS expression in necrotic muscle fibers. Results of HE staining (A–C) and HPGDS immunostaining (D–F) at 6 hour (A, D), 2 days (B, E), and 4 days (C, F) after BPVC injection. Scale bar = 50 μm. Arrows and arrowheads indicate HPGDS-positive infiltrating macrophages and regenerating muscle fibers, respectively. G: Western blotting for HPGDS. H: DP1 expression in necrotic muscle fibers. Left, HE staining; right, DP1 immunocytochemistry. Scale bar = 50 μm. I–L: Quantitative RT-PCR for mRNAs of HPGDS (I), CD11b (J), DP1 (K), and DP2 (L). n = 4. Data are the mean ± SE.
Figure 2
PGD2 overproduction augments muscle necrosis. A: Result of H&E staining of the quadriceps muscle of wild-type and human HPGDS-TG mice obtained at 2 and 4 days after the injection of BPVC. Scale bar = 50 μm. B: Comparison of the water content in the muscle between wild-type and human HPGDS-TG mice at 2 days after the BPVC injection. The water content is shown as the value relative to that of control mice. n = 4 in each group. Data are the mean ± SE. *P < 0.05.
Figure 3
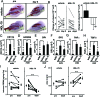
HPGDS inhibition reduces muscular necrosis. A, B: Evans blue dye leakage (A) and expression of CD11b mRNA (B) in the quadriceps muscle from mice in a BPVC-induced model of muscular necrosis treated with or without daily oral applications of the HPGDS inhibitor HQL-79. n = 3. Data are the mean ± SE. *P < 0.05, **P < 0.01. C: Enhanced CT imaging. The three-dimensional images are constructed by summing those sliced CT images along the Z-axis. Arrows indicate enhanced necrotic muscle. D–G: Three-dimensional images of necrosis evaluated by enhanced X-ray CT analyses of the quadriceps muscle of mice with BPVC-induced muscular necrosis treated with vehicle (D), HQL-79 (E), DP1 antagonist (BW A868C, F) or DP2 antagonist (Ramatroban, G). Red spots indicate the penetrated enhancer, and blue color indicates the bone. H: Necrotic muscle volume, n = 6. Data are the mean ± SE. *P < 0.05.
Figure 4
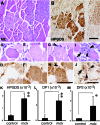
HPGDS expression in the necrotic muscle from an mdx mouse. A, C, E, G, I: H&E staining. B, D, F, H, J: HPGDS immunocytochemistry. Scale bar = 50 μm. Arrowheads indicate HPGDS-positive macrophages; and arrows, HPGDS-positive regenerating muscle fibers. K–M : Quantitative RT-PCR for HPGDS, DP1, and DP2 mRNAs. n = 4. Data are the mean ± SE. *P < 0.05, **P < 0.01.
Figure 5
Reduction in the necrotic muscle volume and inflammation in the muscle from mdx mice by treatment with the HPGDS inhibitor HQL-79. A: Representative three-dimensional imaging of muscle necrosis in mdx mouse before and after treatment with vehicle or HQL-79 for 5 days. light pink: total muscle, red: enhanced muscle. B: Left panel shows changes in the volume of necrosis in individual mdx mice. Right panel summarizes changes in volume of necrosis. Necrotic Vehicle; n = 9, HQL-79; n = 14. C–H: Quantitative RT–PCR for mRNAs of CD11b, HPGDS, DP1, DP2, TNFα, and TGFβ1 in the muscle from wild-type and mdx mice after treatment with vehicle or HQL-79. Data are the mean ± SE. n = 6 in each group. *P < 0.05. **P < 0.01. I: Amount of tetranor-PGDM in the urine of mdx mice before and after HQL-79 administration. n = 12 and n = 11 in vehicle-treated and HQL-79 treated mdx mice, respectively. **P < 0.0003. J: Grip strength test for mdx mice before and after HQL-79 administration. n = 5 in each group. **P < 0.0003.
Similar articles
- [Hematopoietic prostaglandin D synthase inhibitors for the treatment of duchenne muscular dystrophy].
Kamauchi S, Urade Y. Kamauchi S, et al. Brain Nerve. 2011 Nov;63(11):1261-9. Brain Nerve. 2011. PMID: 22068479 Review. Japanese. - Hematopoietic Prostaglandin D Synthase Is Increased in Mast Cells and Pericytes in Autopsy Myocardial Specimens from Patients with Duchenne Muscular Dystrophy.
Hamamura K, Yoshida Y, Oyama K, Li J, Kawano S, Inoue K, Toyooka K, Yamadera M, Matsunaga N, Matsumura T, Aritake K. Hamamura K, et al. Int J Mol Sci. 2024 Feb 3;25(3):1846. doi: 10.3390/ijms25031846. Int J Mol Sci. 2024. PMID: 38339125 Free PMC article. - Protective role of hematopoietic prostaglandin D synthase in transient focal cerebral ischemia in mice.
Liu M, Eguchi N, Yamasaki Y, Urade Y, Hattori N, Urabe T. Liu M, et al. Neuroscience. 2009 Sep 29;163(1):296-307. doi: 10.1016/j.neuroscience.2009.06.027. Epub 2009 Jun 13. Neuroscience. 2009. PMID: 19531375 - Pharmacology and macrophage modulation of HPGDS inhibitor PK007 demonstrate reduced disease severity in DMD-affected muscles of the mdx mouse model.
Yarlagadda S, Sheremeta CL, Cheung SW, Cuffe A, Grounds MD, Smythe ML, Noakes PG. Yarlagadda S, et al. Skelet Muscle. 2025 Apr 24;15(1):11. doi: 10.1186/s13395-025-00379-1. Skelet Muscle. 2025. PMID: 40275384 Free PMC article. - Pharmacological control of cellular calcium handling in dystrophic skeletal muscle.
Ruegg UT, Nicolas-Métral V, Challet C, Bernard-Hélary K, Dorchies OM, Wagner S, Buetler TM. Ruegg UT, et al. Neuromuscul Disord. 2002 Oct;12 Suppl 1:S155-61. doi: 10.1016/s0960-8966(02)00095-0. Neuromuscul Disord. 2002. PMID: 12206810 Review.
Cited by
- "The Social Network" and Muscular Dystrophies: The Lesson Learnt about the Niche Environment as a Target for Therapeutic Strategies.
Cappellari O, Mantuano P, De Luca A. Cappellari O, et al. Cells. 2020 Jul 9;9(7):1659. doi: 10.3390/cells9071659. Cells. 2020. PMID: 32660168 Free PMC article. Review. - Hematopoietic Prostaglandin D Synthase Inhibitor PK007 Decreases Muscle Necrosis in DMD mdx Model Mice.
Yarlagadda S, Kulis C, Noakes PG, Smythe ML. Yarlagadda S, et al. Life (Basel). 2021 Sep 21;11(9):994. doi: 10.3390/life11090994. Life (Basel). 2021. PMID: 34575143 Free PMC article. - Urinary prostaglandin D2 and E2 metabolites are elevated with disease severity in patients with Fukuyama congenital muscular dystrophy.
Ishigaki K, Takeuchi A, Taniguchi-Ikeda M, Sato T, Murakami T, Shichiji M, Ishiguro K, Kihara Y, Nagata S, Urade Y. Ishigaki K, et al. Sci Rep. 2025 Feb 26;15(1):6873. doi: 10.1038/s41598-025-91539-2. Sci Rep. 2025. PMID: 40011677 Free PMC article. - Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis.
Smith WL, Urade Y, Jakobsson PJ. Smith WL, et al. Chem Rev. 2011 Oct 12;111(10):5821-65. doi: 10.1021/cr2002992. Epub 2011 Sep 27. Chem Rev. 2011. PMID: 21942677 Free PMC article. Review. No abstract available. - Sperm and oocyte communication mechanisms controlling C. elegans fertility.
Han SM, Cottee PA, Miller MA. Han SM, et al. Dev Dyn. 2010 May;239(5):1265-81. doi: 10.1002/dvdy.22202. Dev Dyn. 2010. PMID: 20034089 Free PMC article. Review.
References
- Emery AE. Muscular dystrophy into the new millennium. Neuromuscul Disord. 2002;12:343–349. - PubMed
- Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem. 1998;179:111–123. - PubMed
- Kunkel LM, Monaco AP, Hoffman E, Koenig M, Feener C, Bertelson C. Molecular studies of progressive muscular dystrophy (Duchenne). Enzyme. 1987;38:72–75. - PubMed
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. - PubMed
- Manzur AY, Kuntzer T, Pike M, Swan A: Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2004, CD003725 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials