The decision to enter mitosis: feedback and redundancy in the mitotic entry network - PubMed (original) (raw)
Review
. 2009 Apr 20;185(2):193-202.
doi: 10.1083/jcb.200812045. Epub 2009 Apr 13.
Affiliations
- PMID: 19364923
- PMCID: PMC2700378
- DOI: 10.1083/jcb.200812045
Review
The decision to enter mitosis: feedback and redundancy in the mitotic entry network
Arne Lindqvist et al. J Cell Biol. 2009.
Abstract
The decision to enter mitosis is mediated by a network of proteins that regulate activation of the cyclin B-Cdk1 complex. Within this network, several positive feedback loops can amplify cyclin B-Cdk1 activation to ensure complete commitment to a mitotic state once the decision to enter mitosis has been made. However, evidence is accumulating that several components of the feedback loops are redundant for cyclin B-Cdk1 activation during normal cell division. Nonetheless, defined feedback loops become essential to promote mitotic entry when normal cell cycle progression is perturbed. Recent data has demonstrated that at least three Plk1-dependent feedback loops exist that enhance cyclin B-Cdk1 activation at different levels. In this review, we discuss the role of various feedback loops that regulate cyclin B-Cdk1 activation under different conditions, the timing of their activation, and the possible identity of the elusive trigger that controls mitotic entry in human cells.
Figures
Figure 1.
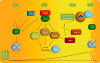
Regulation of cyclin B accumulation. A combination of temporally regulated degradation and transcription ensures that cyclin B levels peak in late G2 phase and during early mitosis. After silencing of the spindle checkpoint in mitosis, cyclin B is degraded by the APC/C. APC/C activity maintains low cyclin B levels through G1 phase until silencing of APC/C in S phase by a combination of direct inactivation by cyclin A–Cdk2, binding of the inhibitor Emi1, and, possibly, autodestruction of the APC/C E2 ligase UbcH10. Starting in S phase and peaking in late G2 phase, accumulating cyclin A–Cdk2 activity also activates the transcription factors B-MYB, NF-Y, and FoxM1, which directly enhance cyclin B transcription. In addition to transcription and degradation, the local concentration of cyclin B is enhanced during centrosome maturation by Plk1- and Aurora A–dependent targeting of cyclin B to the centrosome.
Figure 2.
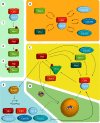
Feedback loops that regulate cyclin B–Cdk1 activity. (A) The inner feedbacks. Myt1 and Wee1 kinases phosphorylate Cdk1 on T14 and Y15, thereby inhibiting cyclin B–Cdk1 activity. The T14 and Y15 phosphorylations can be antagonized by Cdc25A, -B, and -C. Once activated, cyclin B–Cdk1 activity inhibits Wee1 and Myt1, and activates the Cdc25 phosphatases. Thus, by the inner feedback loops, cyclin B–Cdk1 inhibits its inhibitors and activates its activators. (B) Direct Plk1-dependent feedback. Cyclin B–Cdk1 phosphorylation of many targets, including Wee1, Myt1, and Cdc25C, creates a docking site for Plk1. Binding of Plk1 both guides Plk1 to its substrate and stimulates Plk1 activity by releasing its inhibitory polo-box domains. In this way, cyclin B–Cdk1–mediated phosphorylation can trigger a second round of Plk1-mediated phosphorylation of a target protein. (C) Feedback through Bora-Aurora-Plk1. Cyclin B–Cdk1–mediated phosphorylation of Bora increases Bora binding to Plk1. Bora can also associate with Aurora A, and is required for efficient Aurora A–mediated activation of Plk1. By regulating Bora phosphorylation, cyclin B–Cdk1 can thereby activate Plk1. Plk1 in turn activates cyclin B–Cdk1 at different levels (Fig. 2, B, D, and E). Aurora A can also stimulate cyclin B–Cdk1 activation through activation of Cdc25B and regulation of centrosome maturation (Fig. 2 D). Although Aurora A activity is regulated by Cdk, it is currently unclear if these processes depend on cyclin B–Cdk1–mediated phosphorylation of Bora. (D) Feedback through centrosome maturation. The local concentration of cyclin B–Cdk1, an important regulatory step for cyclin B–Cdk1 activation, is enhanced by targeting to the maturating centrosome in late G2 phase. Moreover, many additional proteins in the mitotic entry network are targeted to centrosomes, thereby creating high local concentrations of cyclin B–Cdk1 activators. Both Aurora A and Plk1 activities are required for centrosome maturation and stimulate cyclin B accumulation on centrosomes. (E) Feedback through transcription. Plk1 directly activates the transcription factor FoxM1. FoxM1 stimulates the expression of multiple proteins in the mitotic entry network, including cyclin B. Thus, cyclin B–Cdk1–mediated activation of Plk1 through Bora/Aurora A increases the production of several cyclin B–Cdk1 activators, thereby further stimulating cyclin B–Cdk1 activation.
Figure 3.
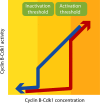
Bistability and hysteresis governing cyclin B–Cdk1 activity. The graph shows the theoretical relationship between cyclin B–Cdk1 concentration and cyclin B–Cdk1 activity. Note that the graph only shows steady-state end points and therefore does not contain any information on how much time is needed to reach the indicated activity. Because of the cyclin B–Cdk1–dependent feedback loops, the majority of cyclin B–Cdk1 complexes are either active, inactive, or approaching one of these states. A major determinant for whether cyclin B–Cdk1 will be active or inactive is the concentration of cyclin B–Cdk1 complexes; cyclin B–Cdk1 activation is triggered above a threshold concentration of cyclin B–Cdk1 complexes (red arrow). However, once the feedback loops are active, they can sustain cyclin B–Cdk1 activity at cyclin B–Cdk1 concentrations below the activation threshold. Therefore, the inactivation threshold is lower than the activation threshold (blue arrow). In this way, the feedback loops provide a resistance to change between the stable states. This resistance ensures that cyclin B–Cdk1 activity, and thereby the decision to enter or exit mitosis, is relatively insensitive to local fluctuations of mitotic entry network components.
Figure 4.
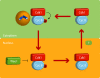
Model of the effect of cyclin B–Cdk1 activity on cyclin B–Cdk1 nucleocytoplasmic shuttling. Once activated, cyclin B–Cdk1 autophosphorylates, thereby increasing its nuclear import. The nuclear import is most likely also affected by other components of the mitotic entry network. In the nucleus, Wee1 levels are high, leading to inactivation of the cyclin B–Cdk1 complex, which can no longer sustain the phosphorylation of cyclin B. After export to the cytoplasm, cyclin B–Cdk1 can be reactivated. Global activation of cyclin B–Cdk1 will occur when enough active cyclin B–Cdk1 enters the nucleus to promote Wee1 degradation, likely in cooperation with inhibitory phosphorylation of the cyclin B nuclear export sequence by an unknown kinase.
Similar articles
- Cyclin A-cdk1-Dependent Phosphorylation of Bora Is the Triggering Factor Promoting Mitotic Entry.
Vigneron S, Sundermann L, Labbé JC, Pintard L, Radulescu O, Castro A, Lorca T. Vigneron S, et al. Dev Cell. 2018 Jun 4;45(5):637-650.e7. doi: 10.1016/j.devcel.2018.05.005. Dev Cell. 2018. PMID: 29870721 - Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited.
Potapova TA, Sivakumar S, Flynn JN, Li R, Gorbsky GJ. Potapova TA, et al. Mol Biol Cell. 2011 Apr 15;22(8):1191-206. doi: 10.1091/mbc.E10-07-0599. Epub 2011 Feb 16. Mol Biol Cell. 2011. PMID: 21325631 Free PMC article. - Cyclin A triggers Mitosis either via the Greatwall kinase pathway or Cyclin B.
Hégarat N, Crncec A, Suarez Peredo Rodriguez MF, Echegaray Iturra F, Gu Y, Busby O, Lang PF, Barr AR, Bakal C, Kanemaki MT, Lamond AI, Novak B, Ly T, Hochegger H. Hégarat N, et al. EMBO J. 2020 Jun 2;39(11):e104419. doi: 10.15252/embj.2020104419. Epub 2020 Apr 30. EMBO J. 2020. PMID: 32350921 Free PMC article. - Spatial control of mitotic commitment in fission yeast.
Hagan IM, Grallert A. Hagan IM, et al. Biochem Soc Trans. 2013 Dec;41(6):1766-71. doi: 10.1042/BST20130190. Biochem Soc Trans. 2013. PMID: 24256289 Review. - PP2A function toward mitotic kinases and substrates during the cell cycle.
Jeong AL, Yang Y. Jeong AL, et al. BMB Rep. 2013 Jun;46(6):289-94. doi: 10.5483/bmbrep.2013.46.6.041. BMB Rep. 2013. PMID: 23790971 Free PMC article. Review.
Cited by
- Replication protein a links cell cycle progression and the onset of neurogenesis in Drosophila optic lobe development.
Zhou L, Luo H. Zhou L, et al. J Neurosci. 2013 Feb 13;33(7):2873-88. doi: 10.1523/JNEUROSCI.3357-12.2013. J Neurosci. 2013. PMID: 23407946 Free PMC article. - Identification of Candidate Cyclin-dependent kinase 1 (Cdk1) Substrates in Mitosis by Quantitative Phosphoproteomics.
Petrone A, Adamo ME, Cheng C, Kettenbach AN. Petrone A, et al. Mol Cell Proteomics. 2016 Jul;15(7):2448-61. doi: 10.1074/mcp.M116.059394. Epub 2016 May 1. Mol Cell Proteomics. 2016. PMID: 27134283 Free PMC article. - Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor.
Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, Kishimoto T. Hara M, et al. Nat Commun. 2012;3:1059. doi: 10.1038/ncomms2062. Nat Commun. 2012. PMID: 22968705 Free PMC article. - Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression.
Lim JM, Lee KS, Woo HA, Kang D, Rhee SG. Lim JM, et al. J Cell Biol. 2015 Jul 6;210(1):23-33. doi: 10.1083/jcb.201412068. J Cell Biol. 2015. PMID: 26150388 Free PMC article. - L-Cystine-Containing Hair-Growth Formulation Supports Protection, Viability, and Proliferation of Keratinocytes.
Riegel K, Hengl T, Krischok S, Schlinzig K, Abts HF. Riegel K, et al. Clin Cosmet Investig Dermatol. 2020 Aug 3;13:499-510. doi: 10.2147/CCID.S254720. eCollection 2020. Clin Cosmet Investig Dermatol. 2020. PMID: 32801826 Free PMC article.
References
- Abrieu A., Brassac T., Galas S., Fisher D., Labbe J.C., Doree M. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs.J. Cell Sci. 111:1751–1757 - PubMed
- Acquaviva C., Pines J. 2006. The anaphase-promoting complex/cyclosome: APC/C.J. Cell Sci. 119:2401–2404 - PubMed
- Alderton G.K., Galbiati L., Griffith E., Surinya K.H., Neitzel H., Jackson A.P., Jeggo P.A., O'Driscoll M. 2006. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling.Nat. Cell Biol. 8:725–733 - PubMed
- Baldin V., Ducommun B. 1995. Subcellular localisation of human wee1 kinase is regulated during the cell cycle.J. Cell Sci. 108:2425–2432 - PubMed
- Baldin V., Pelpel K., Cazales M., Cans C., Ducommun B. 2002. Nuclear localization of CDC25B1 and serine 146 integrity are required for induction of mitosis.J. Biol. Chem. 277:35176–35182 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous