Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability - PubMed (original) (raw)
Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability
Yohei Kirino et al. Nat Cell Biol. 2009 May.
Abstract
Piwi family proteins are essential for germline development and bind piwi-interacting RNAs (piRNAs). The grandchildless gene aub of Drosophila melanogaster encodes the piRNA-binding protein Aubergine (Aub), which is essential for formation of primordial germ cells (PGCs). Here we report that Piwi family proteins of mouse, Xenopus laevis and Drosophila contain symmetrical dimethylarginines (sDMAs). We found that Piwi proteins are expressed in Xenopus oocytes and we identified numerous Xenopus piRNAs. We report that the Drosophila homologue of protein methyltransferase 5 (dPRMT5, csul/dart5), which is also the product of a grandchildless gene, is required for arginine methylation of Drosophila Piwi, Ago3 and Aub proteins in vivo. Loss of dPRMT5 activity led to a reduction in the levels of piRNAs, Ago3 and Aub proteins, and accumulation of retrotransposons in the Drosophila ovary. Our studies explain the relationship between aub and dPRMT5 (csul/dart5) genes by demonstrating that dPRMT5 is the enzyme that methylates Aub. Our findings underscore the significance of sDMA modification of Piwi proteins in the germline and suggest an interacting pathway of genes that are required for piRNA function and PGC specification.
Figures
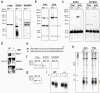
Figure 1. Miwi and Mili proteins contain symmetrical dimethylarginines (sDMAs)
(a) Western blots; 293T(Mili) refers to 293T cells with forced expression of Flag-Mili. (b) Protein immunoprecipitates from mouse testis (NMS: non-immune mouse serum). Mass spectrometry identified Miwi and Mili proteins in the Y12 and Mili protein in the anti-Mili immunoprecipitates (Supplementary Table 1). (h.c) heavy, (l.c) light antibody chain. (c) RNA-immunoprecipitation from mouse testis. **(d)**Arrangement of methyl groups in dimethylarginines. (e) Immunoprecipitates from mouse testis or purified recombinant proteins (Flag-Miwi, Flag-Mili) were probed on Western bots with indicated antibodies. Right panel: coomasie-stained gel of purified recombinant proteins. (f) Full-length or truncated Flag-Miwi (68-862 or 1-212) expressed in 293T cells were immunopurified with anti-Flag and probed with anti-Flag or SYM11 antibodies.
Figure 2. Xenopus laevis Piwi proteins with bound piRNAs are immunoprecipitated by Y12 and contain sDMAs
(a) Protein immunoprecipitates from indicated X. laevis tissues; Xili and Xiwi were identified by mass spectrometry (Supplementary Table 3). (b) Immunoprecipitates from X. laevis oocytes were probed on Western blots with indicated antibodies. Band with asterisk is bovine IgG from tissue culture supernatant of anti-Mili hybridoma. (c) RNA-immunoprecipitations from X. laevis. (d) Periodate oxidation and β-elimination of X. laevis piRNAs isolated from Y12 immunoprecipitates. (e) Nucleotide composition of X. laevis piRNAs. (f) Northern blot for XL-piR-3 (g) In situ hybridization for XL-piR-3 in X. laevis oocyte; bar = 100μm
Figure 3. Drosophila PRMT5 (csul, dart5) is required for arginine methylation of Aub, Piwi and Ago3 proteins in ovaries
(a) Western blots from wild-type (WT) or csul (dPRMT5) mutant (−/−) ovary. Piwi or Aub immunoprecipitates from ovary lysates were probed on western blots with anti-Piwi and anti-Aub antibody (b); or SYM11 and ASYM24 (c). (d) Ago3 immunoprecipitates from WT or csul mutant (−/−) ovary lysates were probed on Western blots (WB) with indicated antibodies. (e) Sequences of wild-type (WT) and mutant (M) Aub, where the four arginines that are substrates for methylation were substituted with lysines. (f) Anti-Flag immunoprecipitates of S2 cells stably transfected with WT or M Flag-Aub were probed on western blots with indicated antibodies. (g) Crosslinking of synthetic, radiolabeled piRNA to immunopurified WT or M Flag-Aub. (h) RNA immunoprecipitation. (i) Periodate oxidation and β-elimination of Drosophila piRNAs isolated from Piwi immunoprecipitates from wt or csul (−/−) mutant ovaries.
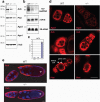
Figure 4. Reduction of Piwi proteins and piRNAs with accumulation of HeT-A retrotransposons and marked reduction of the Aub protein that localizes in the pole plasm, in the absence of dPRMT5 (csul) activity
(a) Western blots of Drosophila ovary lysates from wild-type (wt), heterozygous (+/−) or homozygous (−/−) csul. (b) Northern blots of total RNA from indicated ovary lysates. (c) Fold changes of HeT-A retrotransposon transcripts in indicated ovaries assessed with qRT-PCR; n=3 and s.d. shown. (d) Ago 3, Aub and Piwi localization in indicated early stage egg chambers; bar = 15μm (e) Aub localization in indicated csul oocytes. Arrow indicates pole (germ) plasm; bar = 15μm
Figure 5. Proposed classification for selected Drosophila melanogaster grandchildless genes
Similar articles
- Functional involvement of Tudor and dPRMT5 in the piRNA processing pathway in Drosophila germlines.
Nishida KM, Okada TN, Kawamura T, Mituyama T, Kawamura Y, Inagaki S, Huang H, Chen D, Kodama T, Siomi H, Siomi MC. Nishida KM, et al. EMBO J. 2009 Dec 16;28(24):3820-31. doi: 10.1038/emboj.2009.365. EMBO J. 2009. PMID: 19959991 Free PMC article. - A novel organelle, the piNG-body, in the nuage of Drosophila male germ cells is associated with piRNA-mediated gene silencing.
Kibanov MV, Egorova KS, Ryazansky SS, Sokolova OA, Kotov AA, Olenkina OM, Stolyarenko AD, Gvozdev VA, Olenina LV. Kibanov MV, et al. Mol Biol Cell. 2011 Sep;22(18):3410-9. doi: 10.1091/mbc.E11-02-0168. Epub 2011 Jul 20. Mol Biol Cell. 2011. PMID: 21775629 Free PMC article. - Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization.
Kirino Y, Vourekas A, Sayed N, de Lima Alves F, Thomson T, Lasko P, Rappsilber J, Jongens TA, Mourelatos Z. Kirino Y, et al. RNA. 2010 Jan;16(1):70-8. doi: 10.1261/rna.1869710. Epub 2009 Nov 19. RNA. 2010. PMID: 19926723 Free PMC article. - How does the royal family of Tudor rule the PIWI-interacting RNA pathway?
Siomi MC, Mannen T, Siomi H. Siomi MC, et al. Genes Dev. 2010 Apr 1;24(7):636-46. doi: 10.1101/gad.1899210. Genes Dev. 2010. PMID: 20360382 Free PMC article. Review. - piRNA-mediated silencing in Drosophila germlines.
Siomi MC, Miyoshi T, Siomi H. Siomi MC, et al. Semin Cell Dev Biol. 2010 Sep;21(7):754-9. doi: 10.1016/j.semcdb.2010.01.011. Epub 2010 Jan 18. Semin Cell Dev Biol. 2010. PMID: 20080197 Review.
Cited by
- R-Methylation in Plants: A Key Regulator of Plant Development and Response to the Environment.
Barré-Villeneuve C, Azevedo-Favory J. Barré-Villeneuve C, et al. Int J Mol Sci. 2024 Sep 14;25(18):9937. doi: 10.3390/ijms25189937. Int J Mol Sci. 2024. PMID: 39337424 Free PMC article. Review. - Spatial organization of translation and translational repression in two phases of germ granules.
Ramat A, Haidar A, Garret C, Simonelig M. Ramat A, et al. Nat Commun. 2024 Sep 13;15(1):8020. doi: 10.1038/s41467-024-52346-x. Nat Commun. 2024. PMID: 39271704 Free PMC article. - How germ granules promote germ cell fate.
Pamula MC, Lehmann R. Pamula MC, et al. Nat Rev Genet. 2024 Nov;25(11):803-821. doi: 10.1038/s41576-024-00744-8. Epub 2024 Jun 18. Nat Rev Genet. 2024. PMID: 38890558 Review. - Arabidopsis AGO1 N-terminal extension acts as an essential hub for PRMT5 interaction and post-translational modifications.
Martín-Merchán A, Lavatelli A, Engler C, González-Miguel VM, Moro B, Rosano GL, Bologna NG. Martín-Merchán A, et al. Nucleic Acids Res. 2024 Aug 12;52(14):8466-8482. doi: 10.1093/nar/gkae387. Nucleic Acids Res. 2024. PMID: 38769059 Free PMC article. - Selective degradation of PL2L60 by metabolic stresses‑induced autophagy suppresses multi‑cancer growth.
Sun L, Hui F, Tang GY, Shen HL, Cao XL, Gao JX, Li LF. Sun L, et al. Oncol Rep. 2024 Mar;51(3):41. doi: 10.3892/or.2024.8700. Epub 2024 Jan 12. Oncol Rep. 2024. PMID: 38624021 Free PMC article.
References
- Kim VN. Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev. 2006;20:1993–7. - PubMed
- Hartig JV, Tomari Y, Forstemann K. piRNAs--the ancient hunters of genome invaders. Genes Dev. 2007;21:1707–13. - PubMed
- Harris AN, Macdonald PM. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development. 2001;128:2823–32. - PubMed
- Anne J, Ollo R, Ephrussi A, Mechler BM. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development. 2007;134:137–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS046573/NS/NINDS NIH HHS/United States
- R01 GM076621-01A1/GM/NIGMS NIH HHS/United States
- R01 NS046573/NS/NINDS NIH HHS/United States
- GM0720777/GM/NIGMS NIH HHS/United States
- R01 GM076621/GM/NIGMS NIH HHS/United States
- R01 NS046573-04/NS/NINDS NIH HHS/United States
- R01 GM076621-03/GM/NIGMS NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- R01 GM076621-02/GM/NIGMS NIH HHS/United States
- GM76621/GM/NIGMS NIH HHS/United States
- R01 NS056070/NS/NINDS NIH HHS/United States
- NS056070/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases